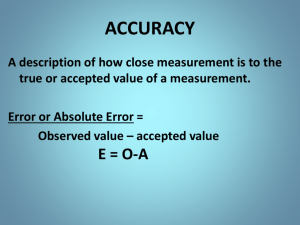
The Metric System The metric System The metric system is an International System of Units (SI Units) used in measurements. All measurements in the metric system are built from base units. The base units are the starting point. We can combine multiple bases to create a larger unit: km is a symbol for kilometers (a measurement of distance) km / h h is a symbol for hours (a measurement of time) Important base units we will use: Base Unit symbol Measures? Metre m Gram g Length and distance Mass Second s Time Litre L Volume Ampere A Electric current Important base units we will use: Base Unit symbol Measures? Metre m Gram g Length and distance Mass Second s Time Litre L Volume Ampere A Electric current We use prefixes to make the base unit bigger or smaller, and we add a symbol to describe its value: Prefix Symbol Multiplying Factor kilo k 1000 hecto h 100 deca da 10 --- --- 1 (base unit) deci d 0.1 centi c 0.01 milli m 0.001 Rules for Changing Metric Units 1. You only move the decimal. The numbers never change. 2. If you are converting to smaller units, move the decimal to the right. When converting to larger units, move the decimal to the left. 3. You must memorize the order of the prefixes to know how many decimal places to move! Ex: Convert 24 g to kg and mg: 24 𝑔 = 0.024 𝑘𝑔 24 𝑔 = 24000 𝑚𝑔 TIP: Use a number line to help organize your prefixes: MOVE DECIMAL RIGHT MOVE DECIMAL LEFT Try These a) 7.6 cm = 76 mm b) 1.43 km = 1430 m c) 0.44 m = 44 cm d) 277 cm = 2.77 m e) 1200 mm = 1.2 m f) 2400 mm = 0.0024 km Note: 1 cm3 = 1 mL Taking precise measurements • Always measure to the precision of the instrument and estimate 1 extra decimal Given by instrument estimated • Ex: 3.24 cm units Ruler tips: Always line the item up at 0 cm Know what units and scale you are using (ex: cm or inches? Divided into 10ths?) Measuring Liquids When measuring liquids, use a graduated cylinder, and read the measurement in line with the bottom of the miniscus (still estimate one extra decimal) To Do… 1. Measurement Stations 2. Metric Conversion Worksheet 3. Measurement Worksheet Measurement, Scientific Notation and Significant Figures Scientific Notation Used in science for very _________ and very ________ numbers Examples: The average distance from the Sun to Earth is 150 000 000 000 m The wavelength of red light is 0.000 000 65 m Using many zeros is inconvenient and can lead to mistakes so scientists came up with an abbreviation called scientific notation Rules 1. 2. 3. 4. 5. Start with the first single digit between 1 and 9 in the number Place a decimal point ( . ) to the right of it After the decimal, write all the other significant figures Multiply the number by 10 Add an exponent to 10 that is equal to the number of spots the decimal has moved Numbers greater than 1 need a positive exponent Numbers less than 1 need a negative exponent Examples First Single Other Significant Figure(s) Digit • 150 000 000 000 m = 1.5 X 1011 m • 0.000 000 65 m = 6.5 X 10 -7 m Significant Figures No measurement is ever perfect or exact Significant figures are all the certain digits plus the first uncertain one Given by instrument estimated 3.24 cm units How many significant figures? Rules for Significant Figures 1. Non-zero digits ARE always significant 2. Zeroes between two significant digits ARE significant (10506: 5 significant figures) 3. Any leading zeroes (0.008 741) ARE NOT significant 4. If there is no decimal point, all the trailing zeroes (75 000) ARE NOT significant 5. Zeroes after decimal points, if shown, are all significant (4.7800 has 5 significant figures) Determining the number of sig. figs # of Sig. Figs 127.3 0.00874o1 0.0050000 3 5 5 Determining the number of sig. figs # of Sig. Figs 127.3 0.00874o1 0.0050000 • 7600: 2 sig. dig. • 0.300: 3 sig. dig. 3 5 5 Hint: Another way to determine the number of significant digits is to convert to scientific notation first and then count the digits Calculations with significant figures Multiplication or division Round the result to the same number of significant figures as the measured value with the least number of significant figures Ex: 2.10 x 0.5896 = 1.23816 Round to 1.24 (three significant digits) Addition or subtraction Round the results to the same number of decimals as the least precise number Ex: 1.586 + 2.31 = 3.896 Round to 3.90 (two decimals) Scientific Inquiry Error Analysis Precision vs. Accuracy Precision and accuracy are two ways that scientists think about error. Accuracy refers to how close a measurement is to the true or accepted value. Precision is about how close measurements of the same item are to each other. Precision and Accuracy are independent of each other. The best scientific observations are Accurate and Precise. Precision vs. Accuracy A: neither accuracy nor precision B: precise, but not accurate C: accurate (because of equal distance from bulls- eye), but not precise D: both accuracy and precision Safety Symbol and Equipment