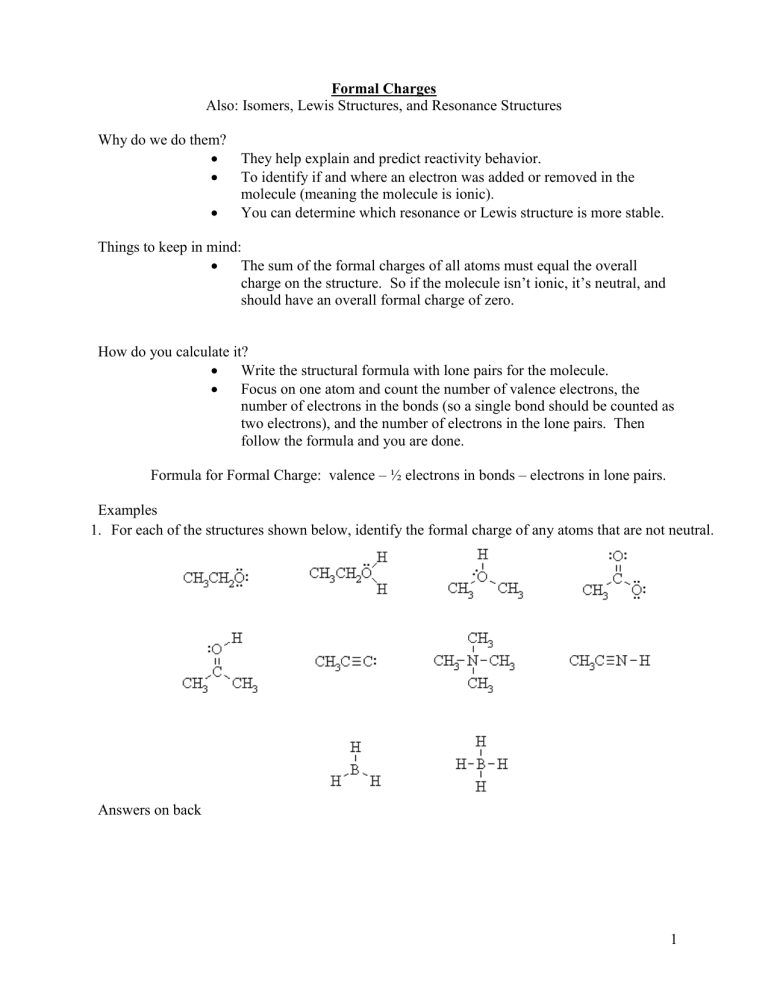
Formal Charges Also: Isomers, Lewis Structures, and Resonance Structures Why do we do them? They help explain and predict reactivity behavior. To identify if and where an electron was added or removed in the molecule (meaning the molecule is ionic). You can determine which resonance or Lewis structure is more stable. Things to keep in mind: The sum of the formal charges of all atoms must equal the overall charge on the structure. So if the molecule isn’t ionic, it’s neutral, and should have an overall formal charge of zero. How do you calculate it? Write the structural formula with lone pairs for the molecule. Focus on one atom and count the number of valence electrons, the number of electrons in the bonds (so a single bond should be counted as two electrons), and the number of electrons in the lone pairs. Then follow the formula and you are done. Formula for Formal Charge: valence – ½ electrons in bonds – electrons in lone pairs. Examples 1. For each of the structures shown below, identify the formal charge of any atoms that are not neutral. Answers on back 1 1. For each of the structures shown below, identify the formal charge of any atoms that are not neutral. Source: http://www.chem.ucalgary.ca/courses/350/Carey5th/Carey.html Formal Charges and Resonance Structures - Ex. NCO 1 Formal charges will be used on the exam to determine which is more stable (and therefore more likely). What you need to consider is the following: 1) Which one has lower formal charges (closer to zero)? The one closest to zero is more stable. 2) Does the negative formal charge get placed on the atom with more electronegativity? Does the positive formal charged get placed on the atom with less electronegativity? If yes, it is more stable. If no, it is less stable. 3) Formal charges of zeroes on atoms next to each other are less stable than atoms that are not adjacent. First structure has the following formal charges: Valence e- ½ e- in bonds e- in lone pairs Formal Charge N 5 3 2 5-3-2 = 0 C 4 4 0 4-4-0 = 0 O 6 1 6 6-1-6 = -1 Total Formal Charge -1+0+0 = -1 Second structure has the following formal charges: Valence e- ½ e- in bonds e- in lone pairs Formal Charge O 6 2 4 6-2-4 = 0 C 4 4 0 4-4-0 = 0 N 5 2 4 5-2-4= -1 Total Formal Charge -1+0+0 = -1 Answer: The first structure is more stable because the negative charge is on the more electronegative atom. 2 Formal Charges and Isomers We will look at only two isomers for CHNO Which structure is more stable? Since the top one has formal charges, the bottom isomer is more stable. None of the atoms have a formal charge, they all equal zero. Difference between Isomers and Resonance Structures Isomers are two or more structural formulas that can be written for the same molecule, except the structures are different (the atoms are moved around). Each isomer is a unique substance with its own physical and chemical properties. Ex. The molecule C3H8O, can be shown correctly in three different ways. Notice the Oxygen is located in a different place. Resonance Structures are a structural formula that indicates the movement of electrons. Neither resonance structure is correct, the truth is somewhere between these structures, meaning the electrons are moving around causing the bonds to shift. Notice, the atoms are NOT moved around. Resonance structures refer to the same substance. Ex. Here the double bond is moving between oxygens. The negatives refer to formal charges. 3 Ex. Here “the lone pairs turn into a double bonds” and vice-versa. This is still a resonance structure since only the electrons are moving. Source: http://preparatorychemistry.com/Bishop_Resonance.htm Remember neither resonance structure truly exists since the electrons are somewhere between these two structures. To indicate this, use dashed-lines. 4