Materials Today: Proceedings 46 (2021) 6087–6090
Contents lists available at ScienceDirect
Materials Today: Proceedings
journal homepage: www.elsevier.com/locate/matpr
Fluorescence resonance energy transfer (FRET) between acriflavine and
CdTe quantum dot
Santanu Chakraborty a,⇑, Syed Arshad Hussain b
a
b
Department of Physics, NIT Agartala, Jirania 799046, India
Thin Film and Nanoscience Laboratory, Department of Physics, Tripura University, Suryamaninagar, 799022 Tripura, India
a r t i c l e
i n f o
Article history:
Received 27 December 2019
Received in revised form 11 February 2020
Accepted 16 February 2020
Available online 14 March 2020
Keywords:
FRET
CdTe
Quantum dot
XRD
a b s t r a c t
In this report we have studied Fluorescence Resonance Energy Transfer between Acriflavine (Acf) and
Cadmium Telluride (CdTe) quantum dot. CdTe quantum dots were synthesized by hydrothermal method.
Spectroscopic characterizations revealed the formation of a FRET pair consisting of Acriflavine (AcF) as
donor and CdTe quantum dot as acceptor having energy transfer efficiency 39.5%.
Ó 2019 Elsevier Ltd. All rights reserved.
Selection and Peer-review under responsibility of the scientific committee of the Third International Conference on Materials Science (ICMS2020).
1. Introduction
Fluorescence resonance energy transfer (FRET) is an electrodynamic phenomenon describing energy transfer between two
light sensitive chromophores [1,2]. It is a distance dependant phenomenon. For successful FRET pair to exist some inevitable conditions must be met, such as donor and the acceptor molecule must
have strong electronic transitions in the visible or UV region of the
spectrum, close proximity between donor and the acceptor molecule is a must, sufficient spectral overlapping between the fluorescence spectrum of donor with the absorption spectrum of the
acceptor, relative orientation between the donor and acceptor
transition dipoles should not be very small and of course the donor
should have high quantum yield [3]. The process of energy transfer
can be of various types depending on the distance between the
donor and acceptor. Within the limit of 1–10 nm distances
between donor and acceptor, FRET is very sensitive whereas at
large distances radiative energy transfer occurs and at distances
less than 1 nm Dexter type energy transfer predominates. So the
above conditions must be fulfilled for a successful FRET pair to
exist. FRET has found enormous applications in different dimensions of science and technology such as mercury sensor [4] nanomedicine [5] cholesterol sensor [6] chemosensor [7] ion sensor
[8] light harvesting systems [9] DNA sensor [10,11] etc. Now a days
⇑ Corresponding author.
quantum dots have found numerous applications in analytical
chemistry. Because of their highly tunable properties, QDs are of
wide interest. Sizes of quantum dots are nano-dimensional due
to the quantum confinement effect. This imparts some unique optical properties to them such as high quantum yields, spectral properties depending on tunable sizes, high fluorescence lifetime,
excellent photostability and broad excitation spectrum [12]. Quantum dots has found wide applications in virus detection [13],
arsenic sensor [14], molecular cell imaging [15], drug delivery
[16] etc. All the properties mentioned above makes quantum dots
a promising candidate to act as a donor molecule in a FRET process
[17]. However, quantum dots are found to be poor acceptors when
combined with donor molecular fluorophores [18]. This is mainly
due to the availability of lower number of molecules in the ground
states. Owing to this despite of good overlapping between the
absorption spectrum of quantum dots and fluorescence spectrum
of donor molecules energy transfer efficiency is less. There are
few reports of quantum dots as acceptors in FRET systems [19–
21]. So, efforts must be given to identify new FRET pairs involving
quantum dots as acceptors and try to increase the energy transfer
efficiency in such a system by optimizing the various parameters
such as experimental conditions, different synthesization procedures, tuning the quantum dot sizes etc. to trade off the optimized
condition. Here in this work we have reported the FRET process
involving Acf as donor molecule and CdTe quantum dot as acceptor
molecule and found that the energy transfer efficiency is 39.5%.
E-mail address: santanu.tu@gmail.com (S. Chakraborty).
https://doi.org/10.1016/j.matpr.2020.02.757
2214-7853/Ó 2019 Elsevier Ltd. All rights reserved.
Selection and Peer-review under responsibility of the scientific committee of the Third International Conference on Materials Science (ICMS2020).
6088
S. Chakraborty, S.A. Hussain / Materials Today: Proceedings 46 (2021) 6087–6090
2. Experimental
The dye AcF was purchased from SigmaChemical Co., USA and
used as received. The chemicals, high purity Cadmium Nitrate
(Cd(NO3)2, 99%), Tellurium powder (Te, 200 mesh, 99.8%),
Sodium Borohydride (NaBH4, 98%), Mercaptopropionic acid (MPA,
C3H6O2S, 99%), and Sodium hydroxide (NaOH) were purchased
from Sigma Aldrich, USA and used without any purification for
the synthesis of CdTe quantum dots. Distilled water was used as
solvent for the synthesis.
The MPA capped CdTe Quantum Dots was synthesized in a
three step procedure using hydrothermal method. The details of
the synthesis procedure are reported elsewhere [22].
Crystal structure of the as prepared quantum dots was characterized by XRD using Bruker D-8 Advance with copper Ka radiation
(wavelength 1.54 Å and 2 hstep of 0.02°. Spectroscopic measurements were carried out using absorption spectrophotometer (PerkinElmer, Lambda 25) and fluorescence spectrophotometer (Perkin
Elmer LS 55).
Table 1
Calculated values of ‘d’ and ‘a’ along with the (hkl) planes.
Peak position (2h)
d (Å)
hkl
a (Å)
24.5
38.20
44.11
63.58
77.18
3.6328
2.3561
2.0529
1.4634
1.2360
111
220
311
331
511
6.29
6.66
6.80
6.37
6.42
the literature [22]. Band-gap energy of the CdTe quantum dots was
found to be 3.65 eV from the Tauc plot (Fig. 2(b)) using the equam
tion ahv ¼ A hv Eg where, a is absorption coefficient, A is optical constant, Eg is optical band gap, h is Planck’s constant, t is
frequency of the incident light, m shows the type of transition, here
m = 2 as CdTe is a direct band gap material. The estimated size of
the synthesized CdTe quantum dot was 2.8 nm, obtained using
Bru’s equation [23]
Eg QD ¼ Eg BULK þ
3. Results and discussion
3.1. Structural charaterization
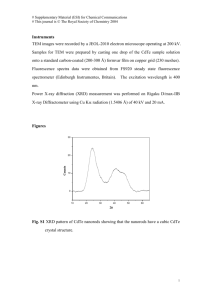
Fig. 1 shows the XRD pattern of the synthesized MPA caped
CdTe QD. The obtained XRD pattern agrees well with the JCPDS
data sheet (2) of CdTe. The 2 h peaks and corresponding assigned
planes (hkl) are marked in the Fig. 1. All these confirm the cubic
zinc blende structure and the lattice is face centred. Intensity of
XRD peak indicates crystalline nature of CdTe quantum dot. The
XRD data interplanar spacing (d) between atoms is calculated
using Bragg’s law 2d Sin h ¼ n ak; where n = 1 and k = 1.5481 Å
wavelength of CuKa. From ‘d’ and (hkl) values, lattice spacing ‘a’
is calculated which is given in the Table 1. According to JCPDS data
sheet the ‘a’ value for CdTe quantum dot is 6.14 Å. However, in our
case some deviation (0.1 to 0.4 Å) from the JCPDS data sheet is
observed which may be attributed due to the change in orientation
by the presence of capping agent MPA.
3.2. Size estimation
Fig. 2(a) shows the Uv–Vis absorption spectrum of the CdTe
quantum dot having a broad peak around 465 nm, consistent with
Fig. 1. X-ray diffraction pattern of CdTe Quantum Dot.
2 h
1
1
1:786e2
þ
8r2 mþe mþh
4pe0 er r
where: EQD
is quantum dots band gap (eV), EBULK
is bulk semicong
g
ductor band gap (eV), r is radius of quantum dot (nm), me* and
mh* are the effective masses of electron and hole respectively, e0
and er are the absolute and relative permittivity respectively, e is
the charge of electron, h is planks constant, for CdTe
ðCdTeÞ
= 1.475 eV, er = 7.1, me* = 0.11 mo, mh* = 0.35mo, where
EBULK
g
mo is absolute mass of electron. However, the actual size of small
crystals is smaller than that obtained from Bru’s equation. In our
case the estimated size 2.8 nm is well below the Bohr exciton radius
of CdTe (7.3 nm) showing the synthesized CdTe quantum dot is
well within quantum confinement region that is of interest for various optical applications.
3.3. FRET study
Fig. 3 shows the normalized absorption and fluorescence spectra of AcF and CdTe quantum dot solutions. Fluorescence spectra of
AcF and CdTe were obtained by exciting the corresponding absorption maxima. The absorption and emission maxima of AcF are centered at 449 nm and 502 nm assigned due to AcF monomers [24].
On the other hand the absorption and emission spectra of CdTe
quantum dots are characterized by peaks centered at 465 nm and
525 nm respectively. Fig. 3 reveals a sufficient spectral overlap
between the fluorescence spectrum of AcF and absorption spectrum of CdTe and also both are highly fluorescent fulfilling the prerequisite for FRET to occur, justifying the selection of the FRET pair,
AcF as donor and CdTe quantum dot as acceptor.
Now, to investigate FRET between AcF and CdTe, we have taken
the fluorescence spectrum of pure AcF, pure CdTe and AcF–CdTe
mixture (1:1 vol ratio) in solution with excitation wavelength fixed
at 420 nm to avoid the direct excitation of the acceptor molecule
(CdTe). Fig. 4 shows the fluorescence spectra of Acf (1), CdTe (2)
and their mixture (Acf–CdTe) (1:1 vol ratio) in aqueous solution.
From figure it was observed that the fluorescence intensity of
AcF (curve 1) is very high whereas that of CdTe (curve 2) negligible
when excited at 420 nm. This is because at this excitation wavelength direct excitation of CdTe was avoided. However in case of
AcF–CdTe mixture (curve 3), the corresponding fluorescence spectra is really very interesting. Here the fluorescence spectrum of AcF
decreases and that of the CdTe increases with respect to their pure
counterpart. This may be due to the transfer of energy from AcF to
CdTe via FRET.
In order to confirm this we have also taken the corresponding
excitation spectra of AcF - CdTe mixture with monitoring emission
S. Chakraborty, S.A. Hussain / Materials Today: Proceedings 46 (2021) 6087–6090
6089
Fig. 2. (a) Uv–Vis absorption spectrum of CdTe quantum dot. (b) Tauc plot of CdTe quantum dot.
wavelength at 502 nm (emission maximum of AcF) and 525 nm
(emission maximum of CdTe) respectively (Fig. 5). Here, both the
excitation spectra were found similar to that of the absorption
spectrum of AcF. This confirm that the origin of transferred energy
in case of AcF–CdTe mixture (as observed in corresponding fluorescence spectra of figure) is AcF, not the direct excitation of CdTe
molecule. This justifies the energy transfer from AcF to CdTe via
FRET.
With the help of Förster Theory [1,2] we have calculated different
FRET
parameters
viz
spectral
overlap
integral
7:62 1015 m1 cm1 nm4 , energy transfer efficiency (39.5%),
Förster radius (6.32 nm) and donor–acceptor distance (6.82 nm)
Fig. 3. The normalized absorption (1 and 3) and emission (2 and 4) spectra of
Acriflavine (Acf) and Cadmium Telluride (CdTe) quantum dot in solution
respectively.
Fig. 4. Fluorescence Spectra of Acf (1), CdTe (2) and their mixture (Acf–CdTe)
(1:1 vol ratio) in aqueous solution.
Fig. 5. Excitation spectra for Acf–CdTe quantum dot (Qdot) mixture monitored with
emission wavelength at 502 nm and 525 nm.
6090
S. Chakraborty, S.A. Hussain / Materials Today: Proceedings 46 (2021) 6087–6090
to quantify the energy transfer from AcF to CdTe. The detail of the
calculation procedure is mentioned elsewhere [3].
ref. No. EMR/2014/000234 and also grateful to UGC, Govt. of India
for financial support to carry out this research work through financial assistance under UGC – SAP program 2016
4. Conclusion
References
In this work we have shown that FRET is possible between AcF
as donor and CdTe quantum dot as acceptor and the different FRET
parameters quantifying energy transfer was also calculated. The
said quantum dot was synthesized by hydrothermal method and
was found to have a size of 2.8 nm which is in the regime appropriate for quantum confinement that is crucial for small crystals to
display various optical applications. XRD analysis reveals highly
crystalline cubic zinc blend type lattice structure of the CdTe quantum dot. Details about the variation of different FRET parameters
with the variation of sizes of quantum dot will be reported in near
future.
CRediT authorship contribution statement
Santanu Chakraborty: Conceptualization, Methodology, Software, Data curation, Writing - original draft, Visualization, Investigation, Supervision, Software, Validation. Syed Arshad Hussain:
Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared
to influence the work reported in this paper.
Acknowledgement
The author S. Chakraborty acknowledges the Department of
Science&Technology, Government of India, for providing UV-Vis
NIR measurement facility through FIST program (SR/FST/PSI196/2014). The authors also acknowledge the Central Research
Facility (CRF) of NIT Agartala for providing XRD measurement facility. The author S. A. Hussain is grateful to DST, for financial support
to carry out this research work through FIST – DST project ref. SR/
FST/PSI-191/2014. SAH is grateful to DST, for financial support to
carry out this research work through DST, Govt. of India project
[1] T.H. Förster, Experimentelle and theoretische untersuchung des Zwis–
chenmolekularen ubergangs von elektrinenanregungsenergie, Z. Naturforsh
4A (1949) 321–327.
[2] T.H. Förster, Transfer mechanisms of electronic excitation, Discuss. Faraday
Soc. 27 (1959) 7–71.
[3] S.A. Hussain, D. Dey, S. Chakraborty, J. Saha, A.D. Roy, S. Chakraborty, P.
Debnath, D. Bhattacharjee, Sci. Lett. J. 4 (2015) 119.
[4] J. Saha, S. Suklabaidya, J. Nath, A.D. Roy, B. Dey, D. Dey, D. Bhattacharjee, S.A.
Hussain, Int. J. Environ. Anal. Chem. (2019) 1–16.
[5] T. Chen, B. He, J. Tao, Y. He, H. Deng, X. Wang, Y. Zheng, Adv. Drug. Delivery 143
(2019) 177–205.
[6] A.D. Roy, D. Dey, J. Saha, P. Debnath, D. Bhattacharjee, S.A. Hussain, Sens.
Actuat. B Chem. 255 (2018) 519–528.
[7] P. Taya, B. Maiti, V. Kumar, P. De, S. Satapathi, Sens. Actuat. B Chem. 255 (2018)
2628–2634.
[8] R. Venkararaj, A. Sarkar, C.P. Girijavallabhan, P. Radhakrishnan, V.P.N.
Nampoori, M. Kailasnath, Appl. Opt. 57 (2018) 4322–4330.
[9] S. Wang, J. Ye, Z. Han, Z. Fan, C. Wang, C. Mu, W. Zhang, W. He, RSC Adv. 5
(2015) 17519–17525.
[10] K. Sapkota, A. Kaur, A. Megalathan, C. Donkoh-Moore, S. Dhakal, Sensors 19
(2019) 3495 (1–13).
[11] A.D. Roy, D. Dey, J. Saha, S. Chakraborty, D. Bhattacharjee, S.A. Hussain,
Spectrochim. Acta A 136 (2015) 1797–1802.
[12] I.L. Medintz, H.T. Uyeda, E.R. Goldman, H. Mattoussi, Nat. Mater. 4 (2005) 435–
446.
[13] S. Huang, H.N. Qiu, Q. Xiao, C.S. Huang, W. Su, B.Q. Hu, J. Fluoresc. 23 (2013)
1089–1098.
[14] G. Tang, J. Wang, Y. Li, X. Su, RSC Adv. 5 (2015) 17519–17525.
[15] D. Deng, L. Qu, S. Achilefu, Y. Gu, Chem. Commun. 49 (2013) 9494–9496.
[16] N.S. Rejinold, T. Baby, S.V. Nair, R. Jayakumar, J. Biomed. Nanotechnol. 9 (2013)
1657–1671.
[17] R. Zekavati, S. Safi, S.J. Hashemi, T. Rahmani-Cherati, M. Tabatabaei, A.
Mohsenifar, M. Bayat, Microchim. Acta 180 (2013) 1217–1223.
[18] A.R. Clapp, I.L. Medintz, J.M. Mauro, B.R. Fisher, M.G. Bawendi, H. Mattoussi, J.
Am. Chem. Soc. 126 (2004) 301–310.
[19] M-K, So1,, A.M., Loening,, S.S., Gambhir,, J., Rao,, Nat. Protoc. 1 (2006)
1160–1164.
[20] H.Q. Yao, Y. Zhang, F. Xiao, Z.Y. Xia, J.H. Rao, Angew. Chem. Int. Ed. 46 (2007)
4346–4349.
[21] D. GeiBler, S. Linden, K. Liermann, K.D. Wegner, L.J. Charbonnière, N.
Hildebrand, Inorg. Chem. 53 (2014) 1824–1838.
[22] B. Jai Kumar, D. Sumanth Kumar, H.M. Mahesh, J. Lumin. 178 (2016) 362–367.
[23] L.E. Brus, J. Chem. Phys. 80 (1984) 4403–4409.
[24] D. Dey, D. Bhattacharjee, S. Chakraborty, S.A. Hussain, Sens. Actuat. B Chem.
184 (2013) 268–273.