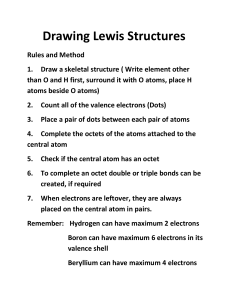
NCEA L2 - Drawing Lewis structures (using the octet rule & duet rule) Lewis formula (electron dot or Lewis structure) Diagrams that show the bonding between atoms of a molecule or polyatomic ion and the lone pairs of electrons that may exist in the molecule or polyatomic ion. One pair of dots represents two electrons a covalent (single) bond or non-bonding pair (lone pair) of electrons Step 1 NH4+ Example H2O Count total # of valence electrons around the atoms, . O=6 (if a polyatomic ion : add 1 e- for each –ve charge or subtract 1 e- for H = 1 each positive charge) H=1 N=5 H=1 H=1 H=1 TOTAL = 8 -1 for + charge TOTAL (9-1) = 8 Step 2 Connect atoms with single bonds H 4 e- s remaining O H H none remaining Step 3 Place remaining e- s in pairs around the atoms , starting with the outer atoms Finished ! O H Step 4 Check valence shell of all atoms are complete (octet). If not move non-bonding pairs to form double or triple bonds Finished ! H H N H + H + Exceptions to octet rule A) Electron deficient molecules E.g. Gaseous BeCl2 and BCl3 Cl Cl Be Cl B Cl Cl Exceptions to octet rule B) Odd electron molecules C) Expanded valence shell F F F F F S F F Xe F F F Cl Cl P Cl Cl Cl Examples Cl • CCl4 Cl C • CO2 Cl • HCN • HOCl • H2O2 H C • PH3 • H2CO (central atom C) • COCl2 (central atom C) • SO2 • OF2 • O3 , NO2+ O C H O P Cl H H O O O H C N H Cl O O H H O F O H O Cl C O F O S Cl O