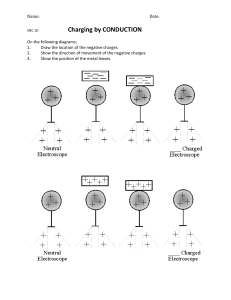
1. Introduction 2. Charges of matter 3. Electric Charges 4. Statement 5. Mathematical Derivation Discovered by a French scientist Charles Augustin De Coulomb. The year of discovery was 1785. Created a device that helped him develop his theories on charge, electric force and field. The device was called Torsion Balance. A matter is said to be charged when there is an imbalance of protons and electrons. The greater is the accumulation of elecrons ,the greater is the charge. Charge is measured in coulombs (C). An electron has 𝟏. 𝟔 × 𝟏𝟎−𝟔 C. Electron is derived from Greek word Elektron meaning amber. Protons have positive charge and electrons have negative charge. Like Charges always repel each other and unlike charges always attract each other. “The Force of attraction or repulsion between any two point charges is directly proportional to the magnitude of the charges and inversely proportional to the square of distance between their centers.” Limitations: The Charges must be point charges ,i.e., small in magnitude and positive. o The charges must be symmetrical and distinct. o Consider a system of two point charges, q1 and q2 , separated by a distance r in vacuum. The force exerted by q1 on q2 is given by Coulomb's law: Then, according to Coulomb`s Law; 𝑓 ∝ 𝑞1 ……..(1) 𝑓 ∝ 𝑞2 ……..(2) 𝑓 ∝ 𝑟 2 ……..(3) Combining (1) , (2) and (3) : 𝑞1 𝑞2 𝑓∝ 2 𝑟 It can also be written as: 𝑞1 𝑞2 𝑓=𝑘 2 𝑟 Where “k” is constant of proportionality called Coulomb`s Contant. 1 𝑘= 4𝜋𝜀° Where 𝜖° is constant of proportionality called permitivity constant. And; 𝜀° = 8.85 × 10−12 𝐶 2 𝑁 −1 𝑚−2 Then; k= 9 × 109 𝑁𝑚2 𝐶 −2 The equation can also be written as: 1 𝑞1 𝑞2 𝐹= 4𝜋𝜀° 𝑟 2 In vector form: 𝐹= 1 𝑞1 𝑞2 ^ ( )𝑟 4𝜋𝜀° 𝑟 2 End