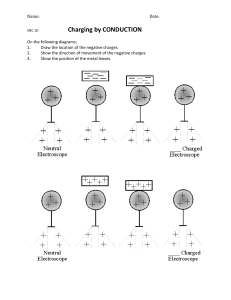
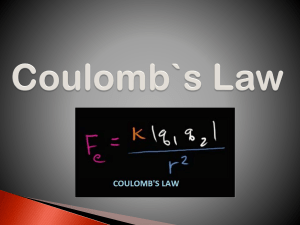
<!--<TO-DETERMINE>--> Force between the charges. <!--</TO-DETERMINE>--> <!--<ANSWER>--> F 1.6 104 N attractive. <!--</ANSWER>--> <!--<EXPLANATION>--> Given: Negative charge, q1 2 104 C Positive charge, q2 8 104 C Distance between the charges, d 0.3 m Formula Used: From Coulomb’s law, F Kq1 q2 d2 Where, F is electrostatic force K is coulomb’s constant q1 and q2 are charges and d is distance between the charges. Calculation: As, K 9 109 N.m2 / C2 On putting the values, F 9 109 2 104 8 104 0.32 1.6 104 N As, F 1.6 104 N Negative means the force is attractive in nature. Conclusion: Therefore, the electrostatic force between the charges is F 1.6 104 N and it is attractive in nature. <!--</EXPLANATION>-->