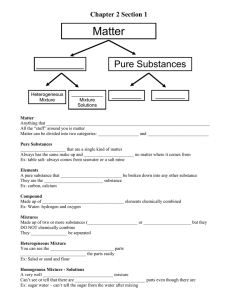
July 19-24 TRY IT YOURSELF CW-29 -To be written in Chemistry copy CW-29 1. What is a substance? State some of its characteristics. 2.How can you say that sodium chloride is a substance but kindness and seawater is not? 3. How can you prove air is a mixture? 4.Is seawater a homogeneous mixture or a heterogeneous mixture? 5.What kind of mixture is (a) sulphur powder & iron filings (b) oil and water (c) soil (d) saline water (h) lemonade (e) chalk powder in water (f) soft drinks (g) steel TRY IT YOURSELF CW-30 -To be written in Chemistry copy CW-30 Answer the following question1.Differentiate between the mixtures and solutions. 2 . Identify the components of given solutions A)air B) Tincture of iodine C) brass 3.Identify the solution based on the number of componentsA) sugar syrup B) Hot coffee C) Gun powder 4.How the solution of common salt in water differs from iodine in alcohol? 5. (1) Alcohol in water is a solution whereas oil in water is not? (2) Sugar in water is a solution but chalk power in water is not CW-31 TRY IT YOURSELF -To be written in Chemistry copy CW-31 1)Write the solute and solvent in the given solutions 1.sea water 2.soda water 3.Bronze 2)Why is air considered as mixture and not a compound? 3) Why sugar /salt in water is called as true solution? 4) What do you understand by solid-solid solution? Explain with an example. 5) Define Solubility? 6) Give an example of (1) gas- liquid solution (2) gas-gas solution.