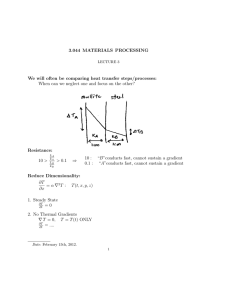
Heat transfer is a science that predicts the transfer of heat energy from one body to another by virtue of temperature difference. 𝑅𝑎𝑡𝑒 𝑜𝑓 𝑇𝑟𝑎𝑛𝑠𝑓𝑒𝑟 𝑃𝑟𝑜𝑐𝑒𝑠𝑠 = 𝑑𝑟𝑖𝑣𝑖𝑛𝑔 𝑓𝑜𝑟𝑐𝑒 𝑟𝑒𝑠𝑖𝑠𝑡𝑎𝑛𝑐𝑒 Heat Transfer • thermal energy in transit due to a spatial temperature difference • Driving force: temperature difference • Quantification of heat transfer processes in terms of appropriate rate equations Mechanism of Heat Transfer Heat transfer occurs as a result of three mechanisms. I. Conduction • heat flows due to molecular interaction, molecules not being displaced or due to the motion free electrons • may be stated as the transfer of internal energy between the molecules. • heat flows by kinetic motion direct impact of molecules whether the body is at rest or in motion in a solid, conduction may be attributed to atomic activity in the form of lattice vibrations • energy transfer to lattice waves induced by atomic motion Rate Equation for Conduction Heat Transfer Processes (Fourier’s Law 𝑞𝑥 𝑑𝑇 = −𝑘 𝐴 𝑑𝑥 𝑊 • • Heat Flux, (𝑚2 ) heat transfer per unit area heat transfer rate in the x direction per unit area perpendicular to the direction of transfer, and it is proportional to the temperature gradient, dT/dx, in this direction • • 𝑊 parameter k (𝑚 ∙ 𝐾) is a transport property known as the thermal conductivity, characteristic of the wall material (find references for thermal conductivities) minus sign is a consequence of the fact that heat is transferred in the direction of decreasing temperature II. Convection - involves the energy exchange between a solid surface and an adjacent fluid. • heat flows or transferred between a fluid and a solid surface as a consequence of motion of fluid particles relative to the solid surface when there exist temperature gradient (occurs between a fluid in motion and a bounding surface when the two are at different temperatures) • two mechanisms: energy transfer due to random molecular motion (diffusion), energy transfer by the bulk, or macroscopic, motion of the fluid Boundary layer development in convection heat transfer. The contribution due to random molecular motion (diffusion) dominates near the surface where the fluid velocity is low. In fact, at the interface between the surface and the fluid the fluid velocity is zero and heat is transferred by this mechanism only. The contribution due to bulk fluid motion originates from the fact that the boundary layer grows as the flow progresses in the x direction. In effect, the heat that is conducted into this layer is swept downstream and is eventually transferred to the fluid outside the boundary layer. Convection classification based on nature of flow: 1. forced convection when the flow is caused by external means, such as by a fan, a pump, or atmospheric winds 2. free (or natural) convection, the flow is induced by buoyancy forces, which are due to density differences caused by temperature variations in the fluid Special cases of convection: there are convection processes for which there is, in addition to sensible heat transfer, latent heat exchange Rate Equation for Convection Heat Transfer Processes (Newton’s law of cooling): 𝑞 = h(𝑇𝑠 − 𝑇𝑓 ) 𝐴 • Heat flux is proportional to the difference between the surface and fluid temperatures 𝑊 𝑚2 • h( • depends on conditions in the boundary layer, which are influenced by surface geometry, the nature of the fluid motion, and an assortment of fluid thermodynamic and transport properties ∙ 𝐾) the convection heat transfer coefficient III. Radiation - The phenomenon or the mode of heat transfer in the form of electromagnetic waves without the presence of any intervening medium • the emission may be attributed to changes in the electron configurations of the constituent atoms or molecules • occurs most efficiently in a vacuum • may also be incident on a surface from its surroundings (Irradiation) • may originate from a special source, such as the sun, or from other surfaces to which the surface of interest is exposed • A portion, or all, of the irradiation may be absorbed by the surface, thereby increasing the thermal energy of the material Rate Equation for Radiation Heat Transfer Processes (Stefan–Boltzmann law): 𝑞 = 𝜀𝜎𝑇𝑠 4 𝐴 • Heat flux is the radiation energy that is emitted by the surface which originates from the thermal energy of matter bounded by the surface • 𝑇𝑠 is the absolute temperature (K) of the surface • 𝜎 is the Stefan–Boltzmann contant, σ = 5.67 × 10−8 𝑊/𝑚2 ∙ 𝐾 4 • 𝜀 is a radiative property of the surface called the emissivity, depends strongly on the surface material and finish • When 𝜀 = 1.0, a surface is called an ideal radiator or blackbody, which provides the maximum heat transfer rate • the range 0 ≤ 𝜀 ≤ 1.0 provides a measure of how efficiently a surface emits energy relative to a blackbody Relevant Properties in the Analysis of Heat Transfer Problems 1. Transport properties • Diffusion rate coefficients • Thermal conductivity coefficients • Kinematic viscosity 2. Thermodynamic properties • Density • Specific heat • Volumetric heat capacity: measures the ability of a material to store thermal energy • Thermal diffusivity: the ratio of the thermal conductivity to the heat capacity