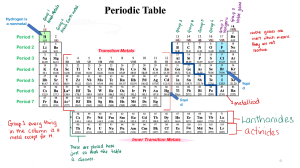
GROUP 1 METALS Through the videos and tasks, you will investigate the physical and chemical properties of the group 1 metals also known as the Alkali Metals Geraldine Fsadni Grade 9 Chemistry ALKALI METALS Reactions of the alkali metals Part A: Investigating the physical properties of the group 1 metals Learning Objective Investigating the physical properties of group 1 metals To establish a trend in the physical of group 1 metals Carry an internet search to complete the table below about the physical properties of the group 1 metals Alkali metal Appearance Melting Point Density Lithium Sodium Potassium 1. Compare the appearance, melting point and density of these metals to metals such as iron and copper ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ 2. How do the melting points and density vary down the group? ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ Page 1 of 11 Reactions of the alkali metals Part B: Investigating the chemical properties of the group 1 metals Learning Objectives To investigate the reactions of group 1 metals with water, chlorine and oxygen To use the above reactions to establish the trend in the reactivity if any down the group Part B 1: Reaction of Alkali metals with water 1 Watch the videos of the reactions of lithium, sodium and potassium with water. Lithium and water Sodium and water Potassium and water Page 2 of 11 Reactions of the alkali metals Write your observations for each reaction in the table below Reaction of lithium with water Reaction of sodium with water Reaction of potassium with water Page 3 of 11 Reactions of the alkali metals a Which was the most reactive? Explain your answer based on what you saw. __________________________________________________________________________________________________________ b What do you think will happen if you put a piece of caesium in water? Explain your answer. ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ 2 There was a flame above the potassium during the reaction with water. What was burning? ________________________________________________________________________________________________________ 3 An indicator was put into the water before the metals were added. a. Name the indicator being used ___________________________________________________________________________________________________________ b. What colour was it before the metals were added? Explain your answer. ___________________________________________________________________________________________________________ c. What happened to the colour as the reaction took place? Explain your answer. ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ Page 4 of 11 Reactions of the alkali metals 4 Copy and complete these word equations to show what happens when lithium and sodium react with water. lithium + water lithium hydroxide + .......................... sodium + ......... .......... ................ + hydrogen This is the balanced equation for the reaction of potassium with water. 2 K (s) + 2 H2O (l) 2 KOH (aq) + H2 (g) 5 Write balanced equations for the reactions of the following alkali metals with water: a lithium ________________________________________________________________________________________________________ b rubidium _______________________________________________________________________________________________________ Page 5 of 11 Reactions of the alkali metals Part B 2: Reaction of Alkali metals with oxygen Watch the following videos of the reactions of alkali metals with oxygen and write your observations in the table below lithium with oxygen sodium with oxygen Potassium with oxygen Page 6 of 11 Reactions of the alkali metals Write your observations and chemical equation for each reaction in the table below Reaction of lithium with oxygen Observation Chemical equation Reaction of sodium with oxygen Observations Chemical equation/s Reaction of potassium with oxygen Observations Chemical equation/s Page 7 of 11 Reactions of the alkali metals Part B3: Reactions of alkali metals with chlorine Watch this video showing the reactions of lithium, sodium and potassium with chlorine Lithium and chlorine Sodium and chlorine Potassium and chlorine Page 8 of 11 Reactions of the alkali metals Write your observations and chemical equation for each reaction in the table below Reaction of lithium with chlorine Observation Chemical equation Reaction of sodium with chlorine Observations Chemical equation/s Reaction of potassium with chlorine Observations Chemical equation/s Page 9 of 11 Reactions of the alkali metals 7a. Draw diagrams to show the atomic structure of lithium, sodium and potassium Lithium Sodium Potassium b. What must an alkali metal do to obtain a full outer shell? c. Which of these metals is likely to obtain a full outer shell most easily? Explain why d. Put the metals of group 1 in order of reactivity starting with the most reactive e. Explain the trend in reactivity by referring to their electronic configuration Page 10 of 11 Reactions of the alkali metals CONCLUSIONS Watch this video which summarises what we have studied above https://www.youtube.com/watch?v=CmiitvJiCPc After watching the video, write concluding remarks about how softness, density, melting point and reactivity changes down the group. ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ Page 11 of 11