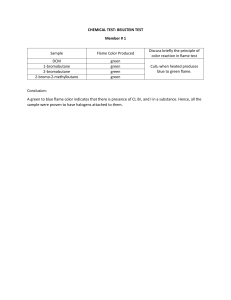
FLAME PHOTOMETRY 1 INTRODUCTION: Theory and Flame spectrometric techniques •Flame photometry (more accurately called Flame Atomic Emission Spectrometry) is a branch of spectroscopy in which the species examined in the spectrometer are in the form of atoms •A photoelectric flame photometer is an instrument used in inorganic chemical analysis to determine the concentration of certain metal ions among them sodium, potassium, calcium and lithium. •Flame Photometry is based on measurement of intensity of the light emitted when a metal is introduced into flame. –The wavelength of colour tells what the element is (qualitative) –The colour's intensity tells us how much of the element present (quantitative) 2 •The basic principle upon which Atomic Spectroscopy works is based on the fact that "Matter absorbs light at the same wavelength at which it emits light". •Atoms of elements subjected to hot flame specific quantum of thermal energy absorbed by orbital electrons become unstable at high energy level release energy as photons of particular wavelength change back to ground state. •When a metal salt solution is burned, the metal provides a colored flame and each metal ion gives a different colored flame. •Flame tests, therefore, can be used to test for the absence or presence of a metal ion 3 PRINCIPLE : •Liquid sample contaning metal salt solution is introduced into a flame, •Solvent is first vaporized, leaving particles of solid salt which is then vaporised into gaseous state •Gaseous molecule dissociate to give neutral atoms which can be excited (made unstable) by thermal energy of flame •The unstable excited atoms emit photons while returning to lower energy state •The measurement of emitted photons forms the basis of flame photometry. 4 • Liquid sample • introduced into a flame • Solvent vaporized • leaving particles of solid salt • vaporized into gaseous state • Gaseous molecule dissociate to give neutral atoms 5 excited (made unstable) by thermal energy of flame The unstable excited atoms emit photons while returning to lower energy state Flame photometry The measurement of emitted photons forms the basis of flame photometry 6 •Under constant and controlled conditions, the light intensity of the characteristic wavelength produced by each of the atoms is directly proportional to the number of atoms that are emitting energy, which in turn is directly proportional to the concentration of the substance of interest in the sample. •Various metals emit a characteristic color of light when heated. 7 Limitation of Flame Emission Photometry •The number of excited atoms in flame is very small. It is the alkaline and alkaline earth metals that can be practically determined. •It needs perfect control of flame temperature. •Interference by other elements is not easy to be eliminated. •Heavy and transition metals , the number of absorption and emission lines is enormous and the spectra are complex. 8 Schematic Representation of the Flame Photometer Major Components: 1. Sample Delivery System 2. flame 3. Monochromator 4.Detector 5.Read out device 9 9 10 11 Nebulizer Flame Burners Mirrors Slits 12 This is the component of sample delivery system. which breaks up the bigger liquid droplet to smaller liquid droplets. The process of conversion of sample to a fine mist of finely divided droplets using a jet of compressed gas is known as Nebulization. 13 Pneumatic nebulizers Electro thermal vaporizer Ultrasound nebulizer 14 CONCENTRIC TUBES FRITTED DISK PNEUMATIC NEBULIZERS CROSS FLOW BAGINGTON 15 The liquid sample is sucked through a capillary tube by a high pressure jet of gas flowing around the tip of the capillary. The high velocity breaks the sample into a mist and carries it to the atomization region. 16 The jet stream flows right angles to the capillary tip. It uses a high speed stream of gas perpendicular to the tip of the sample capillary 17 The jet is pumped through a small orifice in a sphere on which a thin film of sample flows In this type of nebulizer the sample solution flows freely over small aperture, rather than passing through a fine capillary 18 The sample is pumped into a fritted disk through which the gas jet is flowing and this gives fine aerosol than others High efficiencies can be obtained by introducing the sample at predetermined location of the fritted surface 19 It is an electro thermal vaporizer contains an evaporator in a closed chamber through which an inert gas carries the vaporized sample into the atomizer 20 The sample is pumped onto the surface of a vibrating piezoelectric crystal. The resulting mist is denser and more homogeneous than pneumatic nebulizers 21 SAPMLE INTRODUCTION TYPES AND TECHNIQUES 22 Flame and Flame temperature It should have proper temperature Temperature should remain constant throughout the operation There should not be any fluctuation during burning To convert the analyte of the liquid sample into vapour state To decompose the analyte into atoms and simple molecules To excite the formed atoms/free atoms/simple molecules to emit radiant energy 23 24 Structure of Flame: As seen in the figure, the flame may be divided into the following regions or zones. Preheating zones Primary reaction zone or inner zone Internal zone Secondary reaction zone 25 preheating zone- In this, combustion mixture is heated to the ignition temperature by thermal conduction from the primary reaction zone. primary reaction zone- This zone is about 0.1 mm thick at atmospheric pressure There is no thermodynamic equilibrium in this zone and the concentration of ions and free radicals is very high. This region is not used for flame photometry. interconal zone – It can extend up to considerable height. The maximum temperature is achieved just above the tip of the inner zone. This zone is used for flame photometry. secondary reaction zone - In this zone, the products of the combustion processes are burnt to stable molecular species by the surrounding air. 26 Fuel Oxidant Temperature 0C Natural gas Air 1700-1900 Natural gas Oxygen 2700-2800 Hydrogen Air 2000-2100 Hydrogen Oxygen 2550-2700 Acetylene Air 2100-2400 Acetylene Oxygen 3050-3150 Acetylene Nitrous oxide 2600-2800 27 BURNERS Several kinds of burners are used to convert the fine droplets of sample solution into neutral atom ,which further due to the high heat or temperature of flame are excited hence emit radiation of characteristic wavelength and color. Types of burner used: Mekker or Mecker burner Total consumption burner Premix burner Lundergarph’s burner Shielded burner Nitrous oxide – Acetylene burner 28 Mecker burner Nitrous OxideAcetylene Flames Total consumption burner Premix of laminar flow burner Shielded Burner Lundergraph burner 29 This burner was used earlier and employed natural gas and oxygen. Produces relatively low temp. and low excitation energies. This are best used for ALKALI metals only. Now-a-days it is not used. 30 In this burner fuel and oxidant are hydrogen and oxygen gases. Sample solution is aspirated through a capillary by high pressure of fuel and Oxidant and burnt at the tip of burner. Entire sample is consumed. 31 In this type of the burner, aspirated sample, fuel and oxidant are thoroughly mixed before reaching the burner opening and then entering the flame. There is high loss of sample(95%) as large droplets are drained out. 32 In this sample and air is mixed in a chamber, this mixed composition is send to fuel nozzle where it is atomized. Here the sample reaches the flame is only about 5% 33 In this flame was shielded from the ambient atmosphere by a stream of inert gas. Shielding is done to get better analytical sensitivity and quieter flame 34 NITROUS OXIDE ACETYLENE FLAME These flames were superior to other flames for effectively producing free atoms. The drawback of it is the high temperature reduces its usefulness for the determination of alkali metals as they are easily ionized and Intense background emission, which makes the measurement of metal emission very difficult 35 The radiation from the flame is emitted in all the directions in space. Much of the radiation is lost and loss of signal results. A mirror is located behind the burner to reflect the radiation back to the entrance slit of the monochromator. The reflecting surface of the mirror is front-faced. 36 The entrance and exit slits are used before and after the dispersion elements. The entrance slit cuts off most if radiation from the surroundings and allows only the radiation from the flame and the mirror reflection of flame to enter the optical system. The exit slit is placed after the monochromator and allows only the selected wavelength range to pass through the detector 37 MONOCHROMATORS The main of the monochromator is to convert polychromatic light into the monochromatic one The two types of monochromator generally used are as under: 1. Prism : Quartz material is used for making prism, as quartz is transparent over entire region 2. Grating : it employs a grating which is essentially a series of parallel straight lines cut into a plane surface 38 39 Device which converts light energy into electrical signals, that are displayed on readout devices. The following types of detectors are employed in instrumentation of Flame Photometer 1. Barrier layer cell/Photovoltaic cell 2. Photomultiplier tube 4 0 Requirements of an ideal detector:It should give quantitative response. It should have high sensitivity and low noise level. It should have a short response time. It should provide signal or response quantitative to wide spectrum of radiation received. 4 1 BARRIER LAYER CELL or PHOTO VOLTAI CELL It cosistitutes The steel support plate 'A' layer of metallic selenium 'B', which is a few hundredths of a millimetre in thickness. 'C' is a thin transparent electrically-conductive layer, applied by cathodic sputtering. It consists of a metallic base plate like iron or aluminium which acts as one electrode. On its surface, a thin layer of a semiconductor metal like selenium is deposited. Then the surface of selenium is covered by a very thin layer of silver or gold which acts as a second collector tube. When the radiation is incident upon the surface of selenium, electrons are generated at the selenium- silver surface and the electrons are collected by the silver. This accumulation at the silver surface creates an electric voltage difference between the silver surface and the basis of the cell. The photomultiplier tube The principle employed in this detector is that, multiplication of photoelectrons by secondary emission of electrons. It consists of a photo emissive cathode (a cathode which emits electrons when struck by photons of radiation), several dynodes (which emit several electrons for each electron striking them) and an anode. A photon of radiation entering the tube strikes the cathode, causing the emission of several electrons. These electrons are accelerated towards the first dynode (which is 90V more positive than the cathode). The electrons strike the first dynode, causing the emission of several electrons for each incident electron. These electrons are then accelerated towards the second dynode, to produce more electrons which are accelerated towards dynode three and so on. Eventually, the electrons are collected at the anode. By this time, each original photon has produced 106 - 107 electrons. The resulting current is amplified and measured. Photomultipliers are very sensitive to UV and visible radiation. They have fast response times. Detector Photomultiplier Readout device. In the past nearly all spectrophotometer used ammeters or galvanometers. Newer digital devices and printers have now replaced these, and many instruments relay their electrical output directly to computer circuits where calculations are performed, allowing direct reporting of sample concentration. Microprocessor and recorders INTERFERNCES DURING QUANTITAIVE ESTIMATION FOLLOWING TYPES OF INTERFERENCES GENERALLY OCCUR DURING QUANTITAIVE STIMATION 1.SPECTRAL INTERFERENCE 2. CHEMICALINTERFERENCES 3. IONIZATION INTERFERENCES 48 SPECTRAL INTERFERENCES • The first type of interference arises when two elements exhibit spectra, which partially overlap, and both emit radiation at some particular wavelength. eg. - the Fe line at 324.73 nm overlaps with the Cu line at 324.75 nm. SOLUTION:It can overcome either by taking measurements at an alternative wavelength which has no overlap, if available, or by removing the interfering element by extraction. • The second type of spectral interference deals with spectral lines of two or more elements which are close but their spectra do not overlap •SOLUTION:It can be reduced by increasing the resolution of the 38 spectral isolation system. A third type of spectral interference occurs due to the presence of continuous background which arises due to high concentration of salts in the sample, especially of alkali and alkaline earth metals SOLUTION:This type of interference can be corrected by using suitable scanning technique. CHEMICAL INTERFERENCE The chemical interferences arise out of the reaction between different interferens and the analyte . These are of different types: 50 CATION- CATION INTERFERENCE • Due to mutual interferences of cations • These interferences are neither spectral nor ionic in nature • Eg. aluminum interferes with calcium and magnesium. Interference due to oxide formation: It arises due to the formation of stable metal oxide if oxygen is present in the flame 51 CATION- ANION INTERFERENCES • The presence of certain anions, such as oxalate, phosphate, sulphate , in a solution may affect the intensity of radiation emitted by an element, resulting in serious analytical error. • • For example, calcium in the presence of phosphate ion forms a stable substance, as Ca3(PO4) which does not decompose easily, 2 resulting in the production of lesser atoms. 52 IONIZATION INTERFERENCES • high temperature flame may cause ionzation of some of the metal atoms, e.g. sodium Na Na+ + e_ The Na+ ion possesses an emission spectrum of its own with frequencies, which are different from those of atomic spectrum of the Na atom. 53 APPLICATIONS: •To estimate sodium, potassium, calcium, lithium etc. level in sample of serum, urine, CSF and other body fluids. •Flame photometry is useful for the determination of alkali and alkaline earth metals. •Used in determination of lead in petrol. •Used in the study of equilibrium constants involving in ion exchange resins. •Used in determination of calcium and magnesium in cement. 54 Qualitative Analysis •Group I & II i.e Na, K, Li, Mg, Ca, Ba, Strontium Quantitative analysis •Rapid quantitative determination of Group I & II Element wavelength Detection limit Element wavelength Detection limit Al 396 0.5 Pb 406 14 Ba 455 3 Li 461 0.067 Ca 423 0.07 Mg 285 1 Cu 325 0.6 Ni 355 1.6 Fe 372 2.5 Hg 254 2.5 Elements, their characteristic emission wavelengths and detection limits 55 Chatwal & Anand; Instrumental Methods of Chemical Analysis, 5/e 2013, page no- 2.370 to 2.375, Himalaya Publishing House. B.K Sharma; Instrumental Methods of Chemical Analysis, 26/e 2007, page no- 430 to 437, GOEL Publishing House. 56 57