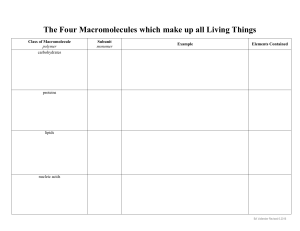
Organic Compounds The Macromolecules of Life: Carbohydrates, Proteins, Lipids, and Nucleic Acids 3. Most Biological Macromolecules are Polymers Polymer: Large molecule consisting of many identical or similar “subunits” linked through covalent bonds. Monomer: “Subunit” or building block of a polymer. Macromolecule: Large organic polymer. Most macromolecules are constructed from about 70 simple monomers. Only about 70 monomers are used by all living things on earth to construct a huge variety of molecules Structural variation of macromolecules is the basis for the enormous diversity of life on earth. Relatively few monomers are used by cells to make a huge variety of macromolecules Macromolecule Monomers or Subunits 1. Carbohydrates 20-30 monosaccharides or simple sugars 2. Proteins 20 amino acids 3. Nucleic acids (DNA/RNA) 4 nucleotides (A,G,C,T/U) 4. Lipids (fats and oils) ~ 20 different fatty acids and glycerol. Making Polymers A. Condensation or Dehydration Synthesis reactions: Process in which one monomer is covalently linked to another monomer (or polymer). The equivalent of a water molecule is removed. Anabolic Reactions: Make large molecules from smaller ones. Require energy (endergonic) General Reaction: Enzyme X - OH + HO - Y --------> Monomer 1 Monomer 2 X - O - Y + H2O Dimer Water (Unlinked) (or Polymer) (or Polymer) Example: Enzyme Glucose + Fructose ---------> Sucrose + (Monomer) (Monomer) (Dimer) H2O Water Breaking Polymers B. Hydrolysis Reactions: “Break with water”. Break down polymers into monomers. Bonds between subunits are broken by adding water. Catabolic Reactions: Break large molecules into smaller ones. Release energy (exergonic) General Reaction: Enzyme X - O - Y + H2O ----------> X - OH + HO - Y Polymer Water Monomer 1 Monomer 2 (or Dimer) Example: Enzyme Sucrose (Dimer) + H2O ---------> Glucose + Fructose Water (Monomer) (Monomer) Synthesis and Hydrolysis of Sucrose IV. Carbohydrates: Molecules that store energy and are used as building materials General Simple Formula: (CH2O)n sugars and their polymers. Diverse group includes sugars, starches, cellulose. Biological Functions: • Fuels, energy storage • Structural component (cell walls) • DNA/RNA component Three types of carbohydrates: A. Monosaccharides B. Disaccharides C. Polysaccharides A. Monosaccharides: “Mono” single & “sacchar” sugar Preferred source of chemical energy for cells (glucose) Can be synthesized by plants from light, H2O and CO2. Store energy in chemical bonds. Carbon skeletons used to synthesize other molecules. Characteristics: 1. May have 3-8 carbons. -OH on each carbon; one with C=0 2. Names end in -ose. Based on number of carbons: 5 carbon sugar: pentose 6 carbon sugar: hexose. 3. Can exist in linear or ring forms 4. Isomers: Many molecules with the same molecular formula, but different atomic arrangement. Example: Glucose and fructose are both C6H12O6. Fructose is sweeter than glucose. Monosaccharides Can Have 3 to 8 Carbons Linear and Ring Forms of Glucose B. Disaccharides: “Di” double & “sacchar” sugar Covalent bond formed by condensation reaction between 2 monosaccharides. Examples: 1. Maltose: Glucose + Glucose. • Energy storage in seeds. • Used to make beer. 2. Lactose: Glucose + Galactose. • Found in milk. • Lactose intolerance is common among adults. • May cause gas, cramping, bloating, diarrhea, etc. 3. Sucrose: Glucose + Fructose. • Most common disaccharide (table sugar). • Found in plant sap. Maltose and Sucrose are Disaccharides C. Polysaccharides: “Poly” many (8 to 1000) Functions: Storage of chemical energy and structure. Storage polysaccharides: Cells can store simple sugars in polysacharides and hydrolyze them when needed. 1. Starch: Glucose polymer (Helical) Form of glucose storage in plants (amylose) Stored in plant cell organelles called plastids 2. Glycogen: Glucose polymer (Branched) Form of glucose storage in animals (muscle and liver cells) Structural Polysaccharides: Used as structural components of cells and tissues. 1. Cellulose: Glucose polymer. The major component of plant cell walls. CANNOT be digested by animal enzymes. Only microbes have enzymes to hydrolyze cellulose, found in digestive systems of: • Cows, goats, and rabbits • Termites 2. Chitin: Polymer of an amino sugar (with NH2 group) Forms exoskeleton of arthropods (insects) Found in cell walls of some fungi Three Different Polysaccharides of Glucose V. Proteins: Large three-dimensional macromolecules responsible for most cellular functions Polypeptide chains: Polymers of amino acids linked by peptide bonds in a specific linear sequence. Protein: Macromolecule composed of one or more polypeptide chains folded into a specific three-dimensional conformation. Proteins have important and varied functions: 1. Enzymes: Catalysis of cellular reactions 2. Structural Proteins: Maintain cell shape 3. Transport: Transport in cells/bodies (e.g. hemoglobin). Channels and carriers across cell membrane. 4. Communication: Chemical messengers, hormones, and receptors. 5. Defensive: Antibodies and other molecules that bind to foreign molecules and help destroy them. 6. Contractile: Muscular movement. 7. Storage: Store amino acids for later use (e.g. egg white). Protein function is dependent upon its 3-D shape. Polypeptide: Polymer of amino acids connected in a specific sequence A. Amino acid: The monomer of polypeptides Central • • • • carbon with: H atom Carboxyl group Amino group Variable R-group Amino Acid Structure: H | (Amino Group) NH2---C---COOH (Carboxyl group) | R (Varies for each amino acid) A Protein’s Specific Shape (Conformation) Determines its Function Conformation: The 3-D structure of a protein. Determined by the amino acid sequence. Four Levels of Protein Structure 1. Primary structure: Linear amino acid sequence, determined by gene for that protein. 2. Secondary structure: Regular coiling/folding of polypeptide. Alpha helix or beta sheet. Caused by H-bonds between amino acids. 3. Tertiary structure: Overall 3-dimensional shape of a polypeptide chain. 4. Quaternary structure: Only found in proteins with 2 or more polypeptides. Overall 3-D shape of all polypeptide chains. Example: Hemoglobin (2 alpha and 2 beta polypeptides) VI. Nucleic acids store and transmit hereditary information for all living things There are two types of nucleic acids in living things: A. Deoxyribonucleic Acid (DNA) Has segments called genes which provide information to make each and every protein in a cell Double-stranded molecule which replicates each time a cell divides. B. Ribonucleic Acid (RNA) Three main types called mRNA, tRNA, rRNA RNA molecules are copied from DNA and used to make gene products (proteins). Usually exists in single-stranded form. DNA and RNA are polymers of nucleotides Nucleic acid: A polymer of nucleotides Nucleotide: Subunits of DNA or RNA. Nucleotides have three components: 1. Pentose sugar (ribose or deoxyribose) 2. Phosphate group to link nucleotides (-PO4) 3. Nitrogenous base (A,G,C,T or U) Purines: Have 2 rings. • Adenine (A) • Guanine (G) Pyrimidines: Have one ring. • Cytosine (C) • Thymine (T) in DNA or uracil (U) in RNA. James Watson and Francis Crick determined the 3D shape of DNA in 1953 Double helix: The DNA molecule is a double helix. Antiparallel: The two DNA strands run in opposite directions. Strand 1: 5’ to 3’ direction (------------>) Strand 2: 3’ to 5’ direction (<------------) Complementary Base Pairing: A & T (U) and G & C. A on one strand hydrogen bonds to T (or U in RNA). G on one strand hydrogen bonds to C. Replication: The double-stranded DNA molecule can easily replicate based on A=T and G=C --- pairing. SEQUENCE of nucleotides in a DNA molecule dictate the amino acid SEQUENCE of polypeptides DNA is a Double Helix Held Together by H-Bonds A Gene is a specific segment of a DNA molecule with information for cell to make one polypeptide DNA (transcribed into single stranded RNA “copy”) ! ! mRNA (single stranded “copy” of the gene) ! ! Polypeptide (mRNA message translated into polypeptide) VII. Lipids: Fats, phospholipids, and steroids Diverse groups of compounds. Composition of Lipids: C, H, and small amounts of O. Functions of Lipids: Biological fuels Energy storage Insulation Structural components of cell membranes Hormones Lipids: Fats, phospholipids, and steroids 1. Simple Lipids: Contain C, H, and O only. A. Fats (Triglycerides). Glycerol : Three carbon molecule with three hydroxyls. Fatty Acids: Carboxyl group and long hydrocarbon chains. Characteristics of fats: Most abundant lipids in living organisms. Hydrophobic (insoluble in water) because nonpolar. Economical form of energy storage (provide 2X the energy/weight than carbohydrates). Greasy or oily appearance. Lipids: Fats, phospholipids, and steroids Simple Lipids: Continued Types of Fats Saturated fats: Hydrocarbons saturated with H. Lack -C=C- double bonds. Solid at room temp (butter, animal fat, lard) Unsaturated fats: Contain -C=C- double bonds. Usually liquid at room temp (corn, peanut, olive oils) Fats (Triglycerides): Glycerol + 3 Fatty Acids 2. Complex Lipids: In addition to C, H, and O, also contain other elements, such as phosphorus, nitrogen, and sulfur. A. Phospholipids: Are composed of: Glycerol 2 fatty acids, Phosphate group Amphipathic Molecule Hydrophobic fatty acid “tails”. Hydrophilic phosphate “head”. Function: Primary component of the plasma membrane of cells B. Steroids: Lipids with four fused carbon rings Includes cholesterol, bile salts, reproductive, and adrenal hormones. Cholesterol: The basic steroid found in animals • • • • Common component of animal cell membranes. Precursor to make sex hormones (estrogen, testosterone) Generally only soluble in other fats (not in water) Too much increases chance of atherosclerosis. C. Waxes: One fatty acid linked to an alcohol. Very hydrophobic. Found in cell walls of certain bacteria, plant and insect coats. Help prevent water loss.