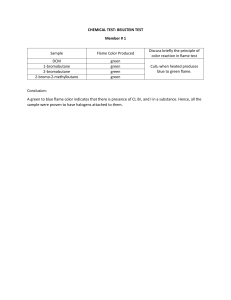
WOLAITA SODO UNIVERSITY COLLEGE OF NATURAL AND COMPUTATIONAL SCIENCES DEPARTMENT OF ENVIRONMENTAL SCIENCE ENVIRONMETAL ANALYTICAL CHEMISTRY (EnSc1052) LAB MANUAL DEGREE: ENVIRONMENTAL SCIENCE SEMESTER II PREPARED BY: Mr., Elias Bojago Editor: Mr., Fekadu T. Wolaita Sodo, Ethiopia Mach, 2021 WOLAITA SODO UNIVERSITY COLLEGE OF NATURAL AND COMPUTATIONAL SCIENCES DEPARTMENT OF ENVIRONMENTAL SCIENCE (ENSC1052) ENVIRONMETAL ANALYTICAL CHEMISTRY LABORATORY Second Semester (Room 3: Environmental Analytical Chemistry Lab) Laboratory Head: Mr. Elias Bojago (email: eliasboja77@gmail.com) Laboratory policy (total weightage: 25% of total course) Laboratory portion: 30% (75% Institute rule applies) Laboratory 25% (10 = lab quiz; 5 = attendance; 10= Reports) Report submission rule: No late submission. Attendance Rule: If a student’s attendance is less than 75%, the student will be awarded one grade less than the actual grade that he (she) has earned. Students needing special accommodations for exams or coursework must submit letters in accordance with institute’s policy three days in advance. Students are responsible for checking IIT Delhi course email list daily for getting course information. All medical reports should be submitted within one week of absence. Outside medical certificate need to be verified at IITD hospital before submission. Study Materials: Some additional power point slides and handouts will be available on the website. Class is the primary place for delivery of materials. Check Teaching Activity Section of Environmental sciences department head office daily for course information) ii Laboratory Guidelines 1) The class will be divided into groups of 4-5 students that will be working together in the lab and in writing the laboratory reports. Each group will submit only one lab report. 2) Always bring lab manual, calculator and lab note book. 3) All lab data will be entered on a sheet of a paper and checked with a Teaching Assistant before attaching it with the lab report. The lab report will be submitted in laboratory itself. 4) All reports should strictly follow this outline (Name/Entry no/Group no.): Title: Include course title, experiment title, date of experiment, date of report submission, and names of group members Summary: A one paragraph statement covering the important objectives, background materials, procedures, results, and conclusions. It is considered to be the most influential parts of reports and it need to be written clearly and concisely. Objectives: Make a clear statement of experimental objectives. Background: In one paragraph explain the importance of the experiment in environmental engineering Methods: Briefly explain the methodology used. Results and Discussion: This section should include a presentation of reduced data (i.e. quantities calculated from raw data) in tabular or graphical format. Refer Tables and Graphs in text and include a title (Table 1, Table 2 or so; Figure 1, Figure 2, or so). Graph axes should be labeled always with units. Conclusions and Recommendations: Briefly list the results and recommendations for improving the experiments. References: Any citations should be documented here. Appendices: Raw data and statistical methods should be included here. Procedure for cleaning of glassware in laboratory Glassware Cleaners Clean the equipment thoroughly with soap and water for basic cleaning. You may need to use a wire brush to remove some residue. Detergent using bottle brushes and scouring pads can be used as needed. iii After cleaning, rinse the glassware with running tap water. When test tubes, graduates, flasks and similar containers are rinsed with tap water, allow the water to run into and over them for a short time, and then partly fill each piece with water. 3. Thoroughly shake and empty at least six times and ensure that all soap residues is removed. Note: • Do not use cleaning brushes that are so worn that the spine hits the glass. Serious scratches may result. Scratched glass is more prone to break during experiments. Any mark in the uniform surface of glassware is a potential breaking point, especially when the piece is heated. Do not allow acid to come into contact with a piece of glassware before the detergent (or soap) is thoroughly removed. If this happens, a film of grease may be formed. • To prevent breakage when rinsing or washing pipets, cylinders or burets, be careful not to let tips hit the sink or the water tap. Sterilizing Contaminated Glassware • Autoclave glassware or sterilize it in large steam ovens or similar apparatus. If viruses or spore bearing bacteria are present, autoclaving is absolutely necessary. Handling and Storing • Protect clean glassware from dust. This is done best by plugging with cotton, corking, taping a heavy piece of paper over the mouth or placing the glassware in a dust-free cabinet. • Store glassware in specially designed racks. Avoid breakage by keeping pieces separated. Why do environmental sampling? • Collection of air, water, soil and dust characterizes the sources of pollution in the environment. • It can be a component of a pollution exposure prevalence study. • It can allow comparison to local and international standards. iv NB many of these standards (e.g. the U.S. Environmental Protection Agency action level for water of 15 parts per billion [ppb]) are pragmatic, meaning they can be reached using good lead control techniques, rather than being health-based. A health-based standard would establish a ‘safe’ level of exposure and this has not been identified for lea Table of Contents Laboratory Guidelines .................................................................................................................................. iii Procedure for cleaning of glassware in laboratory ...................................................................................... iii EXPERIMENT #1 ............................................................................................................................................ 1 Determination of Hardness of Water by EDTA Titrimetric Method ......................................................... 1 EXPERİMENT #2 ............................................................................................................................................ 2 Fluoride (Spands-Zirconium colorimetric method)................................................................................... 2 EXPERİMENT #3 ............................................................................................................................................ 5 Total Phosphorus (Spectrophotometric Method) .................................................................................... 5 Experiment #4 ............................................................................................................................................... 8 Jar Test for Determining Optimum Coagulant Dosage ............................................................................. 8 EXPERIMENT# 5 .......................................................................................................................................... 10 Determination of Total Elements (Fe, Mn, Zn, Cu) ................................................................................. 10 EXPERIMENT# 6 .......................................................................................................................................... 13 Determination of the sodium in water sample (Flame photometric method)....................................... 13 EXPERIMENT# 7 .......................................................................................................................................... 16 Determination of the potassium in given water sample (Flame photometer method) ......................... 16 EXPERIMENT#8 ........................................................................................................................................... 19 Estimation of Amount of Weak Acid....................................................................................................... 19 v vi EXPERIMENT #1 Determination of Hardness of Water by EDTA Titrimetric Method AIM:-To Determine the Hardness of the Given Sample by EDTA Titrimetric Method TITRIMETRIC METHOD PRINCIPLE EDTA and its sodium salt form a compound when added to a solution of certain Metal cations. If a small amount of dye such as Eriochrome black T is added to an aqueous solution containing small calcium and magnesium ions at a pH of a 10±0.50 the solution become wine red. If EDTA is added then Ca and Mg will be complexed. When all these two ions are completed the solution will turn blue. This is the end point of titration. The higher the pH sharper the end point, however above pH10, there is a danger of precipitation of calcium carbonate and magnesium hydroxide .Hence the pH is fixed at 10±0.50, APPARATUS 1. Burette 2. Pipette 3. Erlenmeyer flask REAGENTS Standard EDTA titrant, 0.01 M: Dissolve 3.723 g analytical grade disodium EDTA in dis-tilled water and dilute to 1000 mL. Standardize with CaCO3 solution as follows; Place 25 mL of standard CaCO3 solution into a 250 mL Erlenmeyer flask. Add 2 mL buffer solution and 2 drops of indicator solution. Titrate slowly and carefully with EDTA titrant. The end point is when the color changes from red to blue. Calculate the molarity of standard EDTA titrant. Calculate the mg CaCO3 equivalent to 1 mL EDTA titrant. 1 Standard CaCO3 solution: Weigh 0.25 g of anhydrous CaCO3, powder into a 500 mL Erlenmeyer flask, adds 1+1 HCl drop wise until all the CaCO3 has dissolved. Add 100 mL of distilled water and boil for a few minutes to expel CO2. Cool, add a few drops of methyl red indicator, and adjust to the intermediate orange color by adding 3 N NH4OH or 1+1 HCL, as required. EDTA Solution 0.01M, PROCEDURE 1. Take 20 ml well mixed sample in Erlenmeyer flask 2. Add 1 to 2 ml buffer solution so as to bring the pH to 10+0.50 or 10-0.50 3. Add 2 drops Eriochrome black T indicator solution. The solution turns wine red in colour 4. Titrate against standard EDTA till wine red colour just turns blue. Note down the Volume (v) OBSERVATIONS AND CALCULATIONS Hardness as CaCO3 = (v1xsx1000)/V mg/l Where v1= ml of titrant used S=mg of CaCO3 equivalent to 1ml of EDTA solution=1mg CaCO3 V=volume of sample When reporting hardness, the ions determined should be stated as: hardness (Ca, Mg); hardness (Ca, Mg, Sr, Fe, etc.) and EDTA hardness. Generally, hardness of water is an important consideration in the determining the suitability of a water for domestic and industrial uses. A water sample can be classified as follows: CaCO3, mg/L 0-75 Soft 75-150 Medium 150-300 Hard >300 Very Hard 2 RESULT 1. Hardness as CaCO3 = 3 EXPERİMENT #2 Fluoride (Spands-Zirconium colorimetric method) A .Purpose: To Determine the fluoride present/content in the given water sample. B. Theory/Principle Fluorides in excessive quantities and absence of fluorides in water both create problems. A disfigurement in teeth of humans known as mottled enamel or dental fluorosis is occurred in those, who consume waters with fluoride content in excess of 1.0 mg/L. It has been scientifically established that 0.8-1.0 mg/L of fluorides is essential in potable water. Thus, absence or low fluoride content may cause dental caries in the consumers. Fluorides are measured by colorimetric methods. Fluorides are separated out by distillation, if interfering substances are present. Fluorides are analyzed by a method that involves the bleaching of a performed colour by the fluoride ion. The performed colour is the result of the action between zirconium ion and alizarin dye. The colour produced is referred to a lake and the intensity of colour produced is reduced if the amount of zirconium present is decreased. Fluoride ion combines with zirconium ion to form a stable complex ion ZrF6--and the intensity of the colour lake decreases accordingly. The reaction is as follows: Zr_alizarin lake + 6F (reddish colour) → alizarin + ZrF6--(yellow) The bleaching action is the function of the fluoride ion concentration and is directly proportional to it. Thus, Beer's law is satisfied in and inverse manner. Apparatus Spectrophotometer or colour comparator Reagents Standard fluorides solution 1mL = 10µgF. Acid-zirconyl-alizarin reagent, Zirconyl-alizarin reagent, Sodium arsenite solution Mixed acid solution, Procedure: 1. If residual chlorine is present, remove the same by adding one drop of arsenite per 0.1 mg Cl and mix. 2 2. Prepare a series of standard by diluting various volume of standard fluoride solution (1 ml =10 µgf) to 100 mL in tubes. The range should be such that it is between 0 and 1.4 mg/L. 3. To 50 mL of each standard add 10 mL mixed acid-zirconyl-alizarin reagent. 4. Set the spectrophotometer to a wavelength of 570 nm. 5. Adjust the spectrophotometer to zero absorbance with the reference solution i.e., distilled water with reagent. 6. Plot the concentration along x-axis and absorbance along y-axis and obtain a calibration curve. 7. Take 50 mL of the sample and add 10 mL of mixed acid-zirconyl-alizarin reagent and mix well. 8. Place the solution in the spectrophotometer and read the absorbance. 9. By referring the calibration curve, the concentration for the observed absorbance is read out. 10. Repeat the procedure with dilute samples. Observation The observation is presented in Tables A and B respectively. Table A: Observation for calibration Stock Fluoride solution in mL Fluoride Absorbance 1 2 3 Table B Sample no_ Calculation: F in Absorbance mg L Fluoride in µg from graph A×B = 𝑉×𝐶 A = μg F determined Where, 3 Fluoride in mg B = sample dilute to this volume V = mL of sample. Results: C = portion taken for colour development Sample no_ or Description Fluoride in mg Questions 1. Discuss the significance of high fluorides in water supplies. 2. Discuss the significance of low fluorides in water supplies. 3. What is meant by fluoridation of water? How this can be done? 4. Explain an economical De-fluoridation method for drinking water supplies. 5. What are the various methods for the determination of fluoride in water? 6. Discuss the application of fluoride data. 4 EXPERİMENT #3 Total Phosphorus (Spectrophotometric Method) Aim: Determine the total phosphorus in water sample. Theory/Principle: The method we will use to measure the concentration of pollutants in the water is called Spectrophotometry, which is a procedure that determines how much a chemical compound absorbs and transmits light. Having the light absorbance profile of a solution, we can compare it with a known sample and identify which compounds are present and also their quantities. Spectrophotometry has an immense importance in this scientific community, being one of the most widely used methods for quantifying compounds in different fields such as chemistry, physics, and biology. One of the most common uses of spectrophotometry is the analysis of water samples, which will be explored in this experiment. A spectrophotometer measures the intensity of light that passes through a solution. This is accomplished by shining light at a specific wavelength through a solution and comparing the intensity with a reference blank. Figure 2 depicts a simple spectrophotometer setup. The spectrophotometers in this lab differ slightly from that depicted - a series of LEDs outputting light at specific wavelengths replaces the white light source and monochromator. Fundamentally the mechanism at play is unchanged. Spectrophotometers are only able to emit and detect light within a specific wavelength range and are classified accordingly. For example, a common type is the UV-visible spectrophotometer that emits and absorbs photons with wavelengths in the ultraviolet range (185 nm to 400 nm) and visible range (400 nm to 700 nm), while another type, the IR spectrophotometer, and uses wavelengths in the infrared range (700 nm to 1500 nm). The most accessible of these types is the visible light spectrophotometer, which will be used in this experiment. Particularly for visible light Spectrophotometry, the solutions color can be used as an indicator of the solutions light absorptivity and transmissivity. 5 Apparatus Hot plate, spectrophotometer, glass ware Reagents(a.) Perchloric acid (70%) (b.) Phenolphthalein indicator- Dissolve 1.0 gm of phenolphthalein in 100 ml of ethyl alcohol and add 100 ml of distilled water. (c.) Sodium hydroxide solution (1N) - Dissolve 4 gm of sodium hydroxide in distilled water to prepare 100 ml of solution. (d.) Ammonium molybdate solution- Add 62ml of sulphuric acid (conc.) slowly to 80 ml of distilled water and let cool. Dissolve 5gm of ammonium molybdate in 35 ml of distilled water and mix it with sulphuric acid solution 200 ml. (e.) Stannous chloride solution- Dissolves 0.5gm of stannous chloride in 2ml of conc. HCl and dilute to 20 ml distilled water. (f.) Standard phosphate solution- Dissolve 4.388gm of dried anhydrous potassium hydrogen phosphate in distilled water to make the volume 1liter. Take 10 ml of this solution and add distilled water to make 1 liter of stock solution containing 1 mg P/l. Prepare standard phosphorous solution of various strengths (preferably in the range of 0.0 to 1.0 mg P/l at intervals of 0.1mg P/l) by diluting the stock solution with distilled water. Procedure Take 25 ml of sample in an Erlenmeyer and evaporate to dryness. Cool and dissolve the residue in 1 ml of perchloric acid. Heat the flask gently so that the contents become colorless. Cool and add 10 ml of distilled water and 2 drops of phenolphthalein indicator. Titrate against sodium hydroxide solution until the appearance of slight pink color. Make the volume to 25 ml by addition distilled water. Add 1 ml of ammonium molybdate solution and three drops of stannous chloride solution. A blue color will appear. Wait for 10 minutes (never more than 15 minutes) and record absorbance in spectrophotometer meter at 690 nm. Run simultaneously distilled water blank in similar manner. 6 Process the standard phosphorous solution of different strength (reagent E) in similar manner and plot a standard curve between absorbance and concentration of standard phosphorous solutions. Deduce the total phosphorous content of sample by comparing its absorbance (S) with standard curve and express the result of total phosphorous in mg/l. The total particulate phosphorous can be estimated as a difference between the concentration of total phosphorous in unfiltered and filtered sample. Result- The total phosphorous in given water sample was observed -------mg/l. Precautions1. Glassware should be clean. 2. Prepare the standard solution carefully 7 Experiment #4 Jar Test for Determining Optimum Coagulant Dosage Aim: To determine the optimum coagulant dosage for clarifying the given sample of water by using alum as the coagulant and performing the jar test experiment. Theory/Principle Coagulants are used in water treatment plants To remove natural suspended and colloidal matter, to remove material which does not settle in plain sedimentation, and To assist in filtration. Alum [Al2S(SO4)3. 18H2O] is the most widely used coagulant. When alum solution is added to water, the molecules dissociate to yield SO42- and Al3+. The +ve species combine with negatively charged colloidal to neutralize part of the charge on the colloidal particle. Thus, agglomeration takes place. Coagulation is a quite complex phenomenon and the coagulant should be distributed uniformly throughout the solution. A flash mix accomplishes this. Jar test is simple device used to determine this optimum coagulant dose required. The jar test, device consists of a number of stirrers (4 to 6) provided with paddles. The paddles can be rotated with varying speed with the help of a motor and regulator. Samples will be taken in jars or beakers and varying dose of coagulant will be added simultaneously to all the jars. The paddles will be rotated at 100 rpm for 1 minute and at 40 rpm for 20 to 30 minutes, corresponding to the flash mixing and slow mixing in the flocculator of the treatment plant. After 30 minutes settling, supernatant will be taken carefully from all the jars to measure turbidity. The dose, which gives the least turbidity, is taken as the optimum coagulant dose. Apparatus 1. Jar test apparatus 4. Nephelometer 2. Glass beakers 5. pH meter 3. Pipette Reagents) 8 1. Alum solution (1mL containing 10 mg of alum) 2. Lime 3. Acid/alkali Procedure 1. Take 1-litre beakers and fill them with sample up to the mark. 2. Keep each beaker below each paddle and lower the paddles, such that each one is about 1cm above the bottom. 3. Find the pH of the sample and adjust it to 6 to 8.5. 4. Pipette 1, 2, 3, 4, 5, 6 mL of the alum solution into the test samples. 5. Immediately run the paddles at 100 rpm for 1 minute. 6. Reduce the speed to 30-40 rpm and run at this rate for 30 minutes. 7. Stop the machine, lift out the paddles and allow settling for 30 minutes. 8. Find the residual turbidity of the supernatant using nephelometer. 9. Plot a graph with alum dosage along x-axis and turbidity along y-axis. 10. The dosage of alum, which represents least turbidity, gives Optimum Coagulant Dosage (O.C.D.). 11. Repeat steps 1-10 with higher dose of alum, if necessary. Observation Trial No_ Alum dosage in mg/L Turbidity in NTU Result Optimum coagulant dosage =......... 9 EXPERIMENT# 5 Determination of Total Elements (Fe, Mn, Zn, Cu) Aim: To determine the Total Elements (Fe, Mn, Zn, and Cu) Theory/ principle: Elemental analysis of soils necessitates their decomposition into soluble forms byacid digestion or fusion in Na2CO3. Numerous methods are documented for acid digestion of soil and the determination of elements in solution by atomic absorption spectrometry (1, 3, and 4). The procedure outlined below is an acid digestion (HNO3, HF and HCLO4) and elemental determination by atomic absorption spectrometry. Apparatus /Equipment: Atomic Absorption Spectrometer (IL-257) with background corrector Stainless steel fume hood NOTE: If HClO4 is used in a fume hood that previously was (or later is) used for organic materials, an explosive reaction can occur. Teflon beaker (100 mL capacity) Electric hot plate Volumetric flasks (50 mL capacity) Polypropylene bottles (50 mL capacity) Reagents: Hydrofluoric acid (HF), 48% Per chloric acid (HClO4), 70-72% Nitric acid (HNO3), 10% and 70% solution Water, redistilled or deionized Standard stock solutions (1000 ug/mL) of various elements PROCEDURE 1. Weigh 1.000 g of oven-dry soil, 300 mesh size (0.05 mm sieve opening) into a100 mL Teflon beaker (Weigh 1.000-5.000 g of organic soil). 2. Add 10 mL of conc. HNO3, cover, boil gently for about 30 minutes on a hot late at 100-150˚ C, allow contents to cool. 10 NOTE: Treatment with HNO3 to destroy organic materials is required to prevent danger of an explosion by reaction with HClO4. 3. In a stainless steel fume hood, add 5 mL HClO4 and 15 mL HF, cover, and boil gently for 60 minutes on a hot plate at 150-225˚ C. 4. Remove cover and continue to boil until the volume is reduced to 2-3 mL. NOTE: It is important not to dry the contents completely. 5. Cool and wash down with 5-10 mL of deionized water, cover and bring to a boil for about 20 minutes. NOTE: If a precipitate is present, filter through a What man GF/B filter. Filtration is desirable to keep the solution free of solid particles that cause clogging of burner capillaries of the atomic absorption spectrometer. 6. Wash sample solution into volumetric flask and bring up to volume. Transfer the contents into 100 mL polypropylene bottles for storage (until determination of elements is made). NOTE: It is recommended that a 48-hour soak with 10% HNO3 be used for both the preliminary cleaning of new bottles and for routine cleaning. (4) 7. Prepare standards of elements by dilution of stock solution with the same matrix solution as the sample. 8. Determine elements by atomic absorption spectrometry. NOTE: Prepare and analyze a reagent blank. Calculations ug Reading in ug/mL x 50 in soil = g wt. of sample (g) Reading in ug/mL x 50 %=wt.of sample (g)×10,000 11 Error Values Types n Fe (% 5 Mn (ug/g X/n(mean) SD RSD(%) 1.79 .12 6.7 5 423 16 3.7 Zn (ug/g) 5 54 3 5.6 Cu (ug/g) 5 12 1 8.3 References 1. BAJO, S. 1978. Volatilization of Arsenic (III,V), Antimiony (III.V) and Selenium (IV.VI) from mixtures of hydrogen fluoride and perchloric acid solution; application to silicate analysis. Anal Chem. 50: 649-651. 2. PAWLUK, S. 1967. Soil analyses by atomic absorption spectrophotometry.Atomic absorption Newsletter. 6: 53-56 12 EXPERIMENT# 6 Determination of the sodium in water sample (Flame photometric method) Aim: To determine the amount of sodium in water sample. Principle/ theory: The flame photometer A traditional and simple method for determining sodium and potassium in biological fluids involves the technique of emission flame photometry. This relies on the principle that an alkali metal salt drawn into a non-luminous flame will ionise, absorb energy from the flame and then emit light of a characteristic wavelength as the excited atoms decay to the unexcited ground state. The intensity of emission is proportional to the concentration of the element in the solution. You are probably familiar with the fact that if you sprinkle table salt (NaCl) into a gas flame then it glows bright orange (KCl gives a purple colour). This is the basic principle of flame photometry. A photocell detects the emitted light and converts it to a voltage, which can be recorded. Since Na+ and K+ emit light of different wavelengths (colours), by using appropriate coloured filters the emission due to Na+ and K+(and hence their concentrations) can be specifically measured in the same sample. One drawback of flame photometers, however, is that they respond linearly to ion concentrations over a rather narrow concentration range so suitable dilutions usually have to be prepared. Apparatus: Flame photometer, conical flask etc. Reagents(a.) Stock sodium solution- 2.542 gm dried NaCl is dissolved in distilled water and make up to 1 liter. (1 ml = 1 gm Na) (b.) Working sodium solution- 10 ml of the stock solution is diluted to a1 liter (1 ml = μg Na). 13 Procedure(1.) Start the electrical supply and switch on the air supply, stabilize the air. The needle should be steady at the mark. (2.) Switch on the gas and maintain the gas fuel mixture so that the blue flame is seen through the viewing window. (3.) Aspirate distilled water and adjust the flame photometer reading to zero. (4.) Calibrate the instrument by aspirating the standard and adjusting the flame photometer reading to desired mark by 0-10 and 0-100 mg/l. (5.) Aspirate distilled water to bring the reading to zero mark. (6.) Aspirate sample and note down the flame photometer reading. (7.) Put off the fuel supply first followed by air and then main switch. Result- The sodium in given water sample was observed------------ mg/l. From the information provided on the sachet packaging, calculate the expected concentrations of Na+ ions in the solution you made. [Na+ ] (mM) Concentration How do your own values for Na+ and K+ determined by flame photometry and for Na+ determined with the ISE compare to the expected values? The class data for sodium determination only by both methods will be collected and tabulated on the blackboard in the class. Collect these data in the table below before you leave (12 sets maximum). Using a calculator, calculate the mean and standard deviation (S.D.) for the two data sets and compare these for accuracy and precision. What do you conclude? 14 Type 1 2 3 4 5 6 7 [Na+](flame) [Na+ ] (ISE) Sodium concentration (mean ± S.D, n =) by flame photometry = ……………… (mM) Sodium concentration (mean ± S.D, n =) by ISE = ………………………..…… (mM) Precautions1. Glassware should be clean. 2. Prepare the standard solution carefully. 3. Calibrate instrument properly. 15 8 EXPERIMENT# 7 Determination of the potassium in given water sample (Flame photometer method) Aim: To determine the amount of potassium in water sample Principle/ theory: Potassium (K) is the major cation found inside of cells. The proper level of potassium is essential for normal cell function. An abnormal increase of potassium (hyperkalemia) or decrease of potassium (hypokalemia) can profoundly affect the nervous system and heart, and when extreme, can be fatal. The normal blood potassium level is 3.5 - 5.0 millimoles/liter (mmol/l). Sodium (Na) is the major extracellular cation and it plays a role in body fluid distribution. Concentration of sodium ions inside the plasma (extracellular) is 130-145 mmol/l. Higher and lower concentrations are referred to as hypernatremia and hyponatremia, respectively. When a solution containing cations of sodium and potassium is spayed into flame, the solvent evaporates and ions are converted into atomic state. In the heat of the flame (temperature about 1800ºC), small fraction of the atoms is excited. Relaxation of the excited atoms to the lower energy level is accompanied by emission of light (photons) with characteristic wavelength (Na: 589 nm, K: 766 nm). Intensity of the emitted light depends on the concentration of particular atoms in flame. Apparatus: Flame photometer, plastic bottle, volumetric flask, Desiccator and distilled water. 16 Reagents: (a.) Stock potassium solution- 1.907 gm KCl, dried at 1100C and cooled in desiccator, transfer to 1liter volumetric flask and make to 1 liter with water, (1 ml= 1 mg K). (b.) Intermediate potassium solution- 10 ml stock potassium solution dilute with 100 ml water, (1 ml=0.1 mg K), prepare calibration curve in the range of 1 to 10 mg/l. (c.) Standard potassium solution- Dilute 10 ml intermediate solution with 100 ml water, (1ml= 10 μg K), prepare calibration curve in the range of 0.1 to 1 mg/l. Measurement procedure Attention: Flame photometer uses flammable gases which can cause explosions if used improperly! Switch the instrument on and off under supervision! Note: Check the flame during work if it goes out, close the gas valve immediately! With Eppendorf flame photometers: 1. Let the instrument warm up for 5-10 minutes. 2. Feed distilled water to the instrument. 3. Select the element Na by turning the selector “Elementwahl”. 4. Turn the outer knob “Messbereich” into position “100”. Pull the “Kompensaton I” knob slightly out and adjust readout to 0. Press the “Kompensation I” knob back. Readjust 0 reading with “Kompensation II” if necessary. 5. Aspirate the most concentrated standard solution (solution number 6) and adjust readout to approximately 350 (on uppermost scale) using inner “Messbereich” knob. 6. Aspirate distilled water – the instrument should read 0. 7. Aspirate standard solutions no. 1, 2, 3, test solution, and then standards 4, 5, 6. Record the results. 8. Repeat 3-7 for solutions of potassium. 17 9. Aspirate distilled water for at least 5 minutes to clean the system. With FLAPHO flames photometer: (FLAPHO is a dual channel instrument, which measures concentrations of Na and K simultaneously. Channel 1 (upper indicator) shows Na, and channel 2 (lower) K. 1. Let the instrument warm up for 5-10 minutes. 2. Feed distilled water to the instrument. 3. Using knobs adjust the indicators to 0 reading. 4. Aspirate the most concentrated standard solution (solution number 6) and adjust readout to Approximately 90 (on uppermost scale) using the big knobs. 5. Aspirate distilled water – the instrument should read 0. 6. Aspirate standard solutions no. 1, 2, 3, test solution, and then standards 4, 5, 6. Record the results. 7. Aspirate distilled water for at least 5 minutes to clean the system CalculationMg K/l = mg K from the calibration curve x Dilution Where, Dilution = sample (ml) + distilled water (ml) / Sample (ml Precautions1. Glassware should be clean. 2. Prepare the standard solution carefully. 3. Calibrate instrument properly. 18 EXPERIMENT#8 Estimation of Amount of Weak Acid Aim: To Estimate the Amount of Weak Acid Theory: When weak acid with strong alkali product of reaction titrate is salt of this acid and strong alkali: CH3COOH + NaOH → CH3COONa + H2O Salt hydrolyses: CH3COO− + Na+ + H2O →CH3COOH + Na+ + OH− From (20) equation we can see that [OH−] > [H+]. Because of salt hydrolysis pH>7 in equivalent point (in this case pH= 8.87). We can view pH changes on titration curve. Fig. 1. Weak acid titration with strong alkali curve 19 Color change pH range of phenolphthalein fits to equivalent point, but methyl orange stretches outside titration curve range, so indicator phenolphthalein is suitable to this titration. Remember, when use phenolphthalein solution must be mixed gently, not stirred, because of CO2 present in air, titration error can take place: CO2 + H2O → H2CO3 You can heat solution because solubility of CO2 decreases in higher temperature. Finish titration, when mauve (purplish) color persists for 30 seconds. When titrate weak alkali with strong acid resulting salt hydrolyses, so at equivalent point pH<7. Choose indicator considering this. Task: Titrate solution of weak acid. Using titration results calculate molar equivalent concentration of acid (n), titer (T) and amount of weak acid in solution in grams (m), relative error of analysis. Write a report of analysis. Procedure: Take clean distilled water washed measure flask Write students first and last name, date and student’s journal number on the flask label. Take control sample - unknown amount of weak acid solution. Dilute solution to l00 ml with distilled water, cork it and mix thoroughly. Fill burette with NaOH solution of known concentration. Add l0 ml of solution to three flasks of Erlenmeyer. Add equal amount of indicator phenolphthalein to each flask. Solution must be colorless. Titrate until mauve (purplish) color appears and persists for 30 seconds. Calculate nr using titration data. Calculate relative error R on this data. If R doesn't exceed 5%, calculate titer T and amount of weak acid m (g) Calculate equivalent of organic acid by dividing molecular weight of weak acid by a number of carboxyl groups. Sample Questions to Answer: A water sample has a methyl orange acidity of 60 mg/L. Calculate the quantity of lime in mg/L of Ca (OH) 2 required to raise the pH to 3.7? 20 Reference Materials: AWWA, WEF, APHA, 1998, Standard Methods for the Examination of Water and Wastewater (Methods: 4500 B. Electrometric Method; 2320 B. Titration Method) Sawyer, C.N., McCarty, P.L., and Parkin, G.F. 2000. Chemistry for Environmental Engineering 4th Edition. Tata McGraw-Hill Publishing Company Limited. 21 EXPERIMENT #9 Determination of Chemical Oxygen Demand (COD) Given Water Sample Aim: - To Determine the Chemical Oxygen Demand (COD) Of Given Sample. Introduction: The chemical oxygen demand (COD) test allows measurement of oxygen demand of the waste in terms of the total quantity of oxygen required for oxidation of the waste to carbon dioxide and water. The test is based on the fact that all organic compounds, with a few exceptions, can be oxidized by the action of strong oxidizing agents under acid conditions. Organic matter + Oxidizing agent = CO2 + H2O 12.1 The reaction in Eq.-1 involves conversion of organic matter to carbon dioxide and water regardless of the biological assimilability of the substance. For example, glucose and lignin (biologically inert substance) are both oxidized completely by the chemical oxidant. As a result, COD values are greater than BOD values, especially when biologically resistant organic matter is present. Thus one of the chief limitations of COD test is its inability to differentiate between biodegradable and non-biodegradable organic matter. In addition, it does not provide any evidence of the rate at which the biologically active material would be stabilized under conditions that exist in nature. The major advantage of COD test is the short time required for evaluation. The determination can be made in about 3 hours rather than the 5-days required for the measurement of BOO. For this reason, it is used as a substitute for the BOD test in many instances. Environmental Significance: "COD is often measured as a rapid indicator of organic pollutant in water; it is normally measured in both municipal and industrial wastewater treatment plants and gives an indication of the efficiency of the treatment process. COD has further applications in power plant operations, chemical manufacturing, commercial laundries, pulp & paper mills, environmental studies and general education. Guideline: According to Bangladesh Environment Conservation Rules (1997), drinking water standard for chemical oxygen demand (COD) is 4.0 mg/L. For wastewater effluent allowable 22 concentration of CBOD varies from 200- 400 mg/L depending on discharge point of the effluent (e.g., inland water, irrigation land, public sewer etc.) Principle: Potassium dichromate or potassium permanganate is usually used as the oxidizing agent in the determination of COD. In this class potassium permanganate would be used in the determination of COD. Potassium permanganate is selective in the reaction and attacks the carbonaceous and not the nitrogenous matter. In any method of measuring COD, an excess of oxidizing agent must be present to ensure that all organic matter is oxidized as completely as possible within the power of the reagent. This requires that a reasonable excess be present in all samples. It is necessary, therefore, to measure the excess in some manner so that the actual amount can be determined. For this purpose, a solution of a reducing agent (e.g., ammonium oxalate) is usually used. Apparatus: 1. Beaker (250 mL) 2. Dropper 3. Stirrer Reagent: 1. Diluted sulfuric acid solution 2. Standard potassium permanganate solution 3. Standard Ammonium Oxalate solution Procedure: 1. Pipette 100 mL of the sample into a 250 mL Erlenmeyer flask. 2. Add 10 mL of diluted sulfuric acid and 10 mL of standard KMnO4 solution. 3. Heat the flask in a boiling water bath for exactly 30 minutes, keeping the water in the bath above the level of the solution in the flask. The heating enhances the rate of oxidation reaction in the flask. 23 4. If the solution becomes faintly colored, it means that most of the potassium permanganate has been utilized in the oxidation of organic matter. In such a case, repeat the above using a smaller sample diluted to 100 mL with distilled water. 5. After 30 minutes in the water bath, add 10 mL of standard ammonium oxalate [(NH4)2C2O4] solution into the flask. This 10 mL ammonium oxalate, which is a reducing agent, is just equivalent to the 10 mL potassium permanganate (oxidizing agent) added earlier. The excess of reducing agent [(NH4)2C2O4] now remaining in the flask is just equivalent to the amount of the oxidizing agent (KMn04) used in the oxidation of organic matter 6. The quantity of ammonium oxalate remaining in the flask is now determined by titration with standard potassium permanganate. Titrate the content of the flask while hot with standard potassium permanganate to the first pink coloration. Record the mL of potassium permanganate used. Calculation: COD (mg/L) = DATA SHEET Table Sample No Source of Sample Temperature of Sample (°C) 24 COD (mg/L)