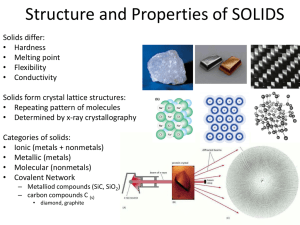
Ch 12 Exercises Classification of Solids (Section 12.1) 12.12 Silicon is the fundamental component of integrated circuits. Si has the same structure as diamond. a. Is Si a molecular, metallic, ionic, or covalent-network solid? Covalent-network solid (see fig 12.1). As in diamond, individual atoms are bound into a three-dimensional network by strong covalent (single) bonds. b. Silicon readily reacts to form silicon dioxide, SiO2, which is quite hard and is insoluble in water. Is SiO2 most likely a molecular, metallic, ionic, or covalent-network solid? Covalent-network solid. The physical properties could describe either an ionic or a covalent-network solid. Silicon and oxygen are both nonmetals, so their bonding is likely to be covalent. 12.14 12.16 Which type (or types) of crystalline solid is characterized by each of the following? a. high mobility of electrons throughout the solid metallic b. softness, relatively low melting point molecular (metallic, as in softer metals) c. high melting point and poor electrical conductivity covalent-network or ionic d. network of covalent bonds covalent-network Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: a. InAs covalent-network b. MgO ionic crystal c. HgS ionic crystal d. In metallic e. HBr molecular 12.18 You are given a white substance that melts at 100 °C. The substance is soluble in water. Neither the solid nor the solution is a conductor of electricity. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be? Molecular. Its physical properties are those of a molecular solid. Structures of Solids (Section 12.2) 12.19 a. Draw a picture that represents a crystalline solid at the atomic level. b. Now draw a picture that represents an amorphous solid at the atomic level. 12.32 For each of these solids, state whether you would expect it to possess metallic properties: a. TiCl4 Not metallic. Compound is ionic. Electrons are localized on individual atoms precluding metallic properties. b. NiCo alloy Metallic. Lattice composed of neutral metal atoms with delocalization of electrons to produce metallic properties. c. W Metallic. Lattice composed of neutral metal atoms with delocalization of electrons to produce metallic properties. d. Ge Not metallic. Ge is a metalloid. e. ScN Not metallic. Compound is ionic. Electrons are localized on individual atoms precluding metallic properties. 12.46 Indicate whether each statement is true or false: a. Intermetallic compounds have a fixed composition. True b. Copper is the majority component in both brass and bronze. True c. In stainless steel, the chromium atoms occupy interstitial positions. False. The chromium atoms are similar in size to iron atoms and form a substitutional alloy. 12.50 Imagine that you have a metal bar sitting half in the sun and half in the dark. On a sunny day, the part of the metal that has been sitting in the sun feels hot. If you touch the part of the metal bar that has been sitting in the dark, will it feel hot or cold? Justify your answer in terms of thermal conductivity. The part of the bar in the dark will feel hot. Delocalized electrons exist over the whole structure of the iron bar. The high thermal conductivity of the metal facilitates heat transfer from the part of the bar in the sun to the part of the bar in the dark. (The reason for these answers goes beyond what you were required to know for this chapter. You can ignore this question.) 12.56 For each of the following groups, which metal would you expect to have the highest melting point: a. gold, rhenium, or cesium rhenium b. rubidium, molybdenum, or indium molybdenum c. ruthenium, strontium, or cadmium ruthenium Ionic and Molecular Solids (Sections 12.5 and 12.6) 12.68 Classify each of the following statements as true or false: False. Melting point is a bulk property, not a molecular property. (The melting point occurs when the intermolecular between the molecules are overcome; the chemical bonds are not broken so their strength is not a factor.) a. For molecular solids, the melting point generally increases as the strengths of the covalent bonds increase. b. For molecular solids, the melting point generally increases as the strengths of the intermolecular forces increase. True. The strength of intermolecular forces determines the properties of the bulk material such as the melting point. Covalent-Network Solids (Section 12.7) 12.70 Which of the following properties are typical characteristics of a covalent-network solid, a metallic solid, or both: a. ductility Metallic solid b. hardness Covalent-network solids and metallic solids (depending on the strength of metallic bonding). c. high melting point Covalent-network solid and metallic solid, depending on the strength of metallic bonding. The extended network of localized covalent bonds in a covalent-network solid produces solids that are inflexible, hard, and high-melting. The delocalized nature of metallic bonding leads to flexibility, because atoms can move relative to one another without breaking bonds. Metals can have a wide range of hardness and melting points/