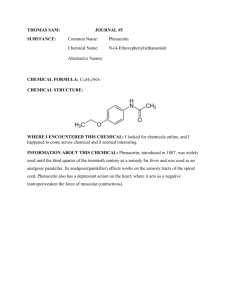
In the Laboratory W Synthesis of the Sweetener Dulcin from the Analgesic Acetaminophen Brian D. Williams,* Birute Williams, and Louise Rodino Department of Chemistry, Kings College, Wilkes-Barre PA 18711; *BDWillia@RS01.Kings.edu Undergraduate organic chemistry students typically show the greatest interest in laboratory experiments utilizing familiar substances or compounds with a definite and readily understood function, such as analgesics, sweeteners, or flavorings. Considering the common use of experiments involving the analgesic aspirin, it is surprising that there have been few synthetic procedures developed for the high school or undergraduate laboratory involving acetaminophen (I) (1–3). This is notable because numerous experiments, concepts, and techniques can be directly linked with acetaminophen and its derivatives and would facilitate integration of its use in a broader pedagogical strategy.1 This report describes the conversion of the analgesic acetaminophen to the artificial sweetener dulcin (IV). The experimental sequence is suitable for both high school and undergraduate laboratories and can be performed on either a micro or macro scale. Phenacetin (II), also a physiologically active analgesic, is an intermediate isolated during the sequence (2, 4 ). Consequently, the procedures described can be adopted as a multistep synthesis of dulcin from acetaminophen or, alternatively as one-period conversions of either acetaminophen to phenacetin or phenacetin to dulcin. A Williamson-type synthesis of aromatic ethers is featured in the former transformation, while the latter demonstrates an amide hydrolysis followed by a nucleophilic addition reaction of an aryl amine with an isocyanate.2 The procedure described offers numerous advantages over previously reported methods, making the experiment suitable for student laboratories. Yields are high and crude products are commonly isolated with sufficient purity that refinement before analysis or use in subsequent reactions is optional. Reaction conditions are optimized to provide adequate time for product purification and characterization within each 3-hour laboratory period. Finally, halogenated solvents are eliminated and the experiment can be run if desired on a single Tylenol tablet, thus significantly minimizing chemical use and wastes. Procedural Overview A well-known procedure for the conversion of I to II involves a 1–3-h reflux of I, ethyl iodide, and K2CO3 catalyst in methyl ethyl ketone followed by several extractions with methylene chloride, producing crude yields ranging from 36 to 61% (2). O O 1. ethanol/NaOH HO N CH3 2. CH3CH2I/reflux CH3CH2CO N H CH3 H (I) (II) HC l/H O/ re x flu 2 O NH3+Cl – CH3CH2CO 1. solid NaHCO3 2. CH3COOH/urea/ reflux CH3CH2CO N NH2 H (III) (IV) The experiment described here involves reaction of I in pure or tablet form with ethyl iodide in an ethanolic NaOH solution. The formation of II is typically complete within a 15-minute reflux period.3 The phenacetin (II) is separated from the insoluble tablet binder by a vacuum filtration into an Erlenmeyer flask that contains a mixture of ice and water. Phenacetin, upon contact with the cold water, will precipitate from the filtrate as a white solid. This procedure simultaneously removes the tablet binder, precipitates the product, and eliminates the use of chlorinated solvents. Students commonly report isolating crude phenacetin in yields between 70 and 90%. A small portion of the sample is reserved for analysis. The product is sufficiently pure to use in the synthesis of IV.4 For the high school laboratory or those not interested in a two-period multistep synthesis, the experiment can be stopped at this point. Otherwise, phenacetin thus produced or commercial phenacetin can be converted into dulcin (IV). Many methods for the preparation of dulcin begin with either p-phenetidine or its salt (6–8). In the procedure described, p-phenetidine hydrochloride salt (III) is formed by hydrolysis of II in excess acid and subsequently converted without isolation into IV, thereby eliminating both isolation and purification of the intermediate p-phenetidine or its salt.5 This is accomplished by careful adjustment of the pH of the p-phenetidine hydrochloride solution with solid portions of NaHCO3 followed by addition of acetic acid and urea and refluxing. The setting of the dulcin into a solid mass indicates completion of the reaction. The mixture is chilled in an ice bath and the dulcin is collected by vacuum filtration as a white crystalline solid. Dulcin formed by this method is typically quite pure and upon drying is suitable for analysis. It can be recrystallized from a minimal amount of hot water. JChemEd.chem.wisc.edu • Vol. 77 No. 3 March 2000 • Journal of Chemical Education 357 In the Laboratory In our laboratories, GC analysis of active reaction mixtures is used to determine adequate reaction times and temperatures, although TLC can also be used.6 The completion of the final reaction is often visible, since the dulcin sets into a solid mass; therefore analysis is not usually required to determine the reaction’s completion. If the monitoring of reactions is not desired, the reaction conditions as reported in the student procedure are adequate. In addition to TLC, GC, melting point, and mixed melting point with genuine samples, product identities can be readily assessed using IR and NMR spectroscopy. Student yields for the conversion of phenacetin to dulcin are reported between 70 and 90%, giving overall yields for the two-step conversion of acetaminophen to dulcin ranging from about 50 to 80%. Discussion The conversion of acetaminophen to dulcin is integrated into our organic laboratory curriculum and is a very popular experiment with our students. The chemical transformations and conversion of “known solids into known solids” is particularly relevant. Students readily comprehend that one of the two substituents on the aromatic ring is modified during each step of the synthesis while the aromatic portion itself remains unaffected. Each product is a white crystalline solid similar to acetaminophen and cannot be differentiated by simple inspection. This provides an excellent opportunity to recognize that changes in just a portion or appendage of a molecule may have little impact on the outward appearance of the substance, while often causing dramatic differences in spectroscopic and physical properties and physiological activity.7 The experimental sequence provides numerous learning opportunities for students by introducing or reinforcing TLC and GC analysis, recrystallization, vacuum filtration, melting point, mixed melting point, IR, NMR, and analysis of active reaction mixtures. Students gain proficiency with chemical calculations, since the quantities of reagents used during the conversion of phenacetin to dulcin must be calculated relative to the amount of phenacetin isolated from Tylenol during the first period. Most students are familiar with acetaminophen. However, they are generally not aware of dulcin because it is no longer used commercially as a sweetener. An examination of sweetener theory (9) or dulcin’s discovery, development, and ultimate removal from the market (10) provides excellent associated reading. This multistep synthesis is preceded in our laboratories by a related sequence that includes the extraction of caffeine from tea, the synthesis of methyl salicylate from aspirin, and the analysis of analgesic mixtures by TLC. Acknowledgments We are grateful to the Juniata College Science Outreach program and The Camille and Henry Dreyfus Foundation for their support and to Juniata College, Wilkes University, and King’s College for the use of their facilities. 358 W Supplemental Material Supplemental material for this article is available in this issue of JCE Online. Notes 1. For example, an experiment involving TLC analysis of analgesic mixtures can be performed before this experiment and students can use the information obtained to follow the progress of the reaction (11). Work is in progress on a problem-based collaborative learning project that incorporates the synthesis described here as part of a greater sequence. 2. The reactive isocyanate is isocyanic acid that is generated in situ by an acid-catalyzed elimination of ammonia from urea. 3. The appropriate amount of base can be calculated or titrated using thymol blue indicator (5). 4. Analysis of crude phenacetin by GC–MS occasionally revealed trace amounts of p-ethoxy aniline. 5. Alternatively, the phenetidine hydrochloride salt can be isolated as a stable solid by precipitating in an ice bath before addition of NaHCO3. 6. In the interest of time, GC analysis was performed only on a few reaction mixtures and the results were discussed with the class and usually taken to be representative. Efforts were made to have each student or group contribute a sample for GC analysis at some time during one of the two periods. A HP model 6890 gas chromatograph equipped with a 30-meter HP-5 column was used for analysis. 7. For example, for the conversion of acetaminophen to phenacetin, the melting points of the two compounds differ by about 32 °C. In addition, the 1H NMR spectra differ dramatically. Phenacetin shows a well-defined ethyl quartet and triplet from the ethoxy substituent, whereas acetaminophen does not. Literature Cited 1. Pavia, D. L.; Lampman, G. M.; Kriz, G. S.; Engel, R. G. Introduction to Organic Laboratory Techniques; Saunders: Philadelphia, 1990; pp 62–66. 2. Volker, E. J.; Pride, E.; Hough, C. J. Chem. Educ. 1979, 56, 831. 3. Rothenberger, O.; Bunting, R.; Newton, T. J. Chem. Educ. 1991, 68, 502–503. 4. Meyers, F. H.; Jawetz, E.; Goldfien, A. Review of Medical Pharmacology, 6th ed.; Lange Medical Publications: Los Altos, CA, 1978; p 288. 5. Ditts, K.; Durand, M. J. Chem. Educ. 1990, 67, 74. 6. Kurzer, F. Org. Synth. Coll. Vol. IV, 1963, 52. 7. Wilcox, Ch. F. Jr. Experimental Organic Chemistry; Macmillan: New York, 1988; p 453. 8. Furnixx, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. Vogel’s Practical Organic Chemistry, 5th ed.; Wiley: New York, 1989; p 966. 9. Ellis, J. W. J. Chem. Educ. 1995, 72, 670–675. 10. Goldsmith, R. H. J. Chem. Educ. 1987, 64, 954–955. 11. Lieu, V. T. J. Chem. Educ. 1971, 48, 478–479. Journal of Chemical Education • Vol. 77 No. 3 March 2000 • JChemEd.chem.wisc.edu