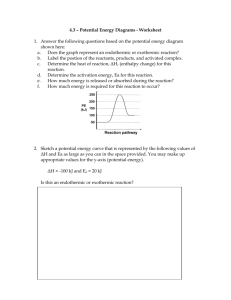
General Chemistry Unit 8 – Equilibrium Name: _________________________ April 12, 2021 Review Guide Section 1 - General 1. Dynamic Equilibrium a. explain in your own words: _______________________________________________________________. b. use the word bank to write the technical definition Dynamic Equilibrium is where _____________________ _____________________ are occurring at the _________________ _________________ and at the ________________ ___________________. Word bank: time rate processes smashing opposing same (note, one word is used twice, another isn’t used) 2. An exothermic reaction ___________________ heat. a. Which side of the reaction is “Heat” written? _____________ b. What happens to the reaction when the temperature is increased? ________________________________ 3. An endothermic reaction __________________heat. a. Which side of the reaction is “Heat” written? _____________ b. What happens to the reaction when the temperature is increased? ________________________________ 4. What happens when the temperature is increased on a reversible chemical reaction that is neither exothermic or endothermic? Section 2 - Equilibrium in a Reversible Chemical Reaction 5. Referring to the following general chemical equation: aA + bB cC + dD a. what do the lower case letters represent? b. what do the upper case letters represent? c. why do we write a double arrow? d. what do we call A and B?(reactants or products) e. what do we call C and D? (reactants or products) f. this arrow represents the _______________________ _________________________ g. this arrow represents the _______________________ _________________________ 1 General Chemistry Unit 8 - Review Guide Section 3 - Le Chatelier’s Principle 6. Le Chatelier’s Principle a. explain in your own words: _______________________________________________________________. b. use the word bank to write a technical definition According to Le Chatelier’s Principle, a reversible chemical reaction at _______________________ will respond to a ___________________ by ___________________ the reaction in a way that relieves the stress. Word bank: shifting stress huge equilibrium (note, one word is not used) Three ways of “stressing” a reversible chemical reaction are: changing the ___________________ of the system (if the reaction is exothermic or endothermic) changing the ___________________ of one of the reactants or products changing the ___________________ of the system (if there are an “unequal” number of moles of gases on both sides of the reaction) 7. Recall the lab we did involving NO2 and N2O4 in a sealed test tube. 2NO2 Brown N2O4 + Colorless heat Predict the color change to the gas in the test tube when… a. the pressure is increased b. the pressure is decreased c. heat is added (test tube put in a hot water bath) d. heat is removed (test tube put in a cold water bath) more brown more brown more brown more brown less brown less brown less brown less brown 8. For each reaction below, state whether the reaction will shift right, shift left, or not shift if we increase the pressure of on the system and defend your answer with a brief explanation. a. NH3(g) + 2O2(g) HNO3(l) + H2O(l) b. CO2(g) + CF4(g) 2COF2(g) c. C(s) + H2O(g) CO(g) + H2(g) 2 General Chemistry Unit 8 - Review Guide Section 4 – Dissolving and Equilibrium 9. Dissolving is a chemical/physical process. (circle the correct answer) 10. Draw a model of NaCl dissolving into water. Show the three “phases” a. concentration = 0 b. concentration is > 0 but less than the saturation point, and c. the solution is saturated. Section 5 – Cobalt-Chloride Lab Reversible chemical reaction: Co(H2O)62+ + Cl- <--> CoCl42- + H2O Available Chemicals: 12M HCl H2O AgNO3(aq) Explain how you were able to determine the color of the cobalt ions (Co(H2O)62+ and CoCl42-). Explain how were you able to determine if the reaction is endothermic or exothermic in the forward direction. Explain why adding AgNO3 caused a shift to occur? 3 General Chemistry Unit 8 - Review Guide Unit 8 – Equilibrium Chemical Reactions o Irreversible or completion o Reversible Reaction Rates o Forward o Reverse Equilibrium o Chemical Equilibrium - Static o Chemical Equilibrium - Dynamic o Non-chemistry examples of dynamic equilibrium Exothermic vs Endothermic Reactions o Definitions o HEAT on the reactant side or product side Le Chatelier’s Principle o Definition o 3 ways to stress Dissolving o 3 phases o Dynamic equilibrium (saturated solutions) Cobalt-Chloride Ion Lab o How was the color of Co(H2O)62+ and CoCl42- determined o How was exothermic or endothermic determined 4