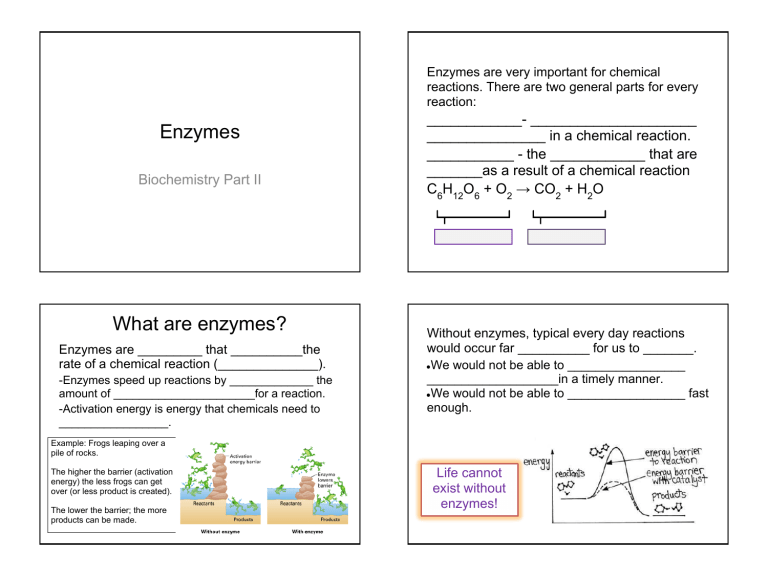
Enzymes are very important for chemical reactions. There are two general parts for every reaction: Enzymes Biochemistry Part II What are enzymes? Enzymes are _________ that __________the rate of a chemical reaction (______________). -Enzymes speed up reactions by _____________ the amount of ______________________for a reaction. -Activation energy is energy that chemicals need to _________________. ____________- _____________________ _______________ in a chemical reaction. ___________ - the ____________ that are _______as a result of a chemical reaction C6H12O6 + O2 → CO2 + H2O Without enzymes, typical every day reactions would occur far __________ for us to _______. ●We would not be able to _________________ ___________________in a timely manner. ●We would not be able to _________________ fast enough. Example: Frogs leaping over a pile of rocks. The higher the barrier (activation energy) the less frogs can get over (or less product is created). The lower the barrier; the more products can be made. Life cannot exist without enzymes! Examples of Enzymes ■Carbonic anhydrase: found in ________; _______________________from our bodies. ■Catalase: found in _______ tissue; speeds up the ______________________________ in our bodies . ■Amylase; found in _______; speeds up the _______________________ for digestion. Enzyme Substrate (the Reactant) Enzyme-Substrate Complex Once the enzyme-substrate complex is formed, the enzyme ________________ slightly to create a more secure bond, and then the chemical reaction follows. The active site is simply where the substrate bonds to the enzyme. Enzyme-Substrate Complex: The Lock and Key -The ___________ of an enzyme is a molecule that ________________________________________. -Enzymes and substrates are _________to each other like a _______________. (For example, the catalase substrate (hydrogen peroxide) would not attach to amylase). Once the substrate and enzyme bond, it becomes the “________________ _______________”. After the chemical reaction, the _______ (former substrate) is __________ from the enzyme. The enzyme is free to carry on the same chemical reaction again and again (enzymes can be _________). Factors that Affect Enzymes Chemical Reactions involving Enzymes -Two factors that affect enzyme productivity are __ and ______________. -Enzymes thrive best within certain temperature and pH _________. -If the ____________ is altered, the _________ ___________ may _____________. Example) people die of fevers not because of the high temperatures, but because the enzymes in their body do not function properly once the temperature gets too high. Chemical Reactions Involving Enzymes www.biology-roots.com In this example, _________________________ ___________________. More specifically, the disaccharide maltose (the substrate) bonds to the enzyme amylase to form two glucose molecules. This is the breakdown of sugar- you’ ll experience it during lunch. www.biology-roots.com In the example above, ___________________ react to combine into a __________________ Enzymes



