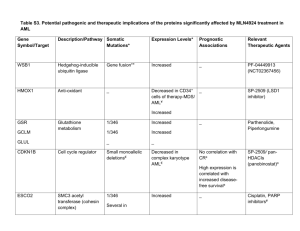
Leukemia (2017) 31, 1482–1490
© 2017 Macmillan Publishers Limited, part of Springer Nature. All rights reserved 0887-6924/17
www.nature.com/leu
REVIEW
Measurable residual disease testing in acute myeloid leukaemia
CS Hourigan1, RP Gale2, NJ Gormley3, GJ Ossenkoppele4 and RB Walter5,6,7
There is considerable interest in developing techniques to detect and/or quantify remaining leukaemia cells termed measurable or,
less precisely, minimal residual disease (MRD) in persons with acute myeloid leukaemia (AML) in complete remission defined by
cytomorphological criteria. An important reason for AML MRD-testing is the possibility of estimating the likelihood (and timing) of
leukaemia relapse. A perfect MRD-test would precisely quantify leukaemia cells biologically able and likely to cause leukaemia
relapse within a defined interval. AML is genetically diverse and there is currently no uniform approach to detecting such cells.
Several technologies focused on immune phenotype or cytogenetic and/or molecular abnormalities have been developed, each
with advantages and disadvantages. Many studies report a positive MRD-test at diverse time points during AML therapy identifies
persons with a higher risk of leukaemia relapse compared with those with a negative MRD-test even after adjusting for other
prognostic and predictive variables. No MRD-test in AML has perfect sensitivity and specificity for relapse prediction at the cohortor subject levels and there are substantial rates of false-positive and -negative tests. Despite these limitations, correlations between
MRD-test results and relapse risk have generated interest in MRD-test result-directed therapy interventions. However, convincing
proof that a specific intervention will reduce relapse risk in persons with a positive MRD-test is lacking and needs testing in
randomized trials. Routine clinical use of MRD-testing requires further refinements and standardization/harmonization of assay
platforms and results reporting. Such data are needed to determine whether results of MRD-testing can be used as a surrogate end
point in AML therapy trials. This could make drug-testing more efficient and accelerate regulatory approvals. Although MRD-testing
in AML has advanced substantially, much remains to be done.
Leukemia (2017) 31, 1482–1490; doi:10.1038/leu.2017.113
INTRODUCTION
He will manage the cure best who has foreseen what is to happen
from the present state of matters.
Hippocrates. The Book of Prognostics. Around 400 BCE.
Complete remission, defined as o5 percent myeloblasts in the
bone marrow determined by cytomorphology and recovery of
blood counts has been the end point for evaluating chemotherapy efficacy in acute myeloid leukaemia (AML) for 60 years.1
Choice of this end point (and not partial remission or stable
disease as is common in lymphomas and solid neoplasms) was
based on two observations. First, persons achieving a complete
remission lived longer than those with any other response.
Second, their increase in survival corresponded directly with
duration of complete remission.2 The latter observation proved
achieving complete remission translated directly into a durable
benefit and was not merely a prognostic variable for longer
survival.
However, there are several limitations of defining remission by
cytomorphology. One is imprecision in quantifying myeloblasts in
bone marrow samples using light microscopy related to the
survey of relatively few (typically 100–400) nucleated bone
marrow cells and intra- and inter-observer variability in identifying
myeloblasts. Another issue is our imperfect ability to distinguish
normal from leukaemia (or preleukaemic) myeloblasts by cytomorphological criteria. A third issue is variability in the distribution
of myeloblasts at different sites.3 Given these limitations it is not
surprising many or most persons with AML in morphological
complete remission relapse. But others, also in complete remission
defined by these criteria, do not relapse. Why? One possibility is all
leukaemia cells able to cause relapse were eradicated by therapy.
Another is some or even many leukaemia cells able to cause
relapse remain but simply do not do so within the observation
interval. There are also other possibilities.
Limitations of defining complete remission by cytomorphology
make it desirable to try to develop more sensitive techniques to
detect residual leukaemia cells, especially those able to cause
leukaemia relapse. But why? Is it to detect some or all residual
leukaemia cell(s), only leukaemia cells biologically able to cause
relapse within a specified interval or which actually cause relapse
within this interval or some other reason? These are distinct,
sometimes overlapping, but not identical goals. As such, the goal
of a technique developed to detect residual leukaemia cells
missed by cytomorphology must be clearly defined.
There are 10–13 × 109/kg nucleated bone marrow cells in a
normal adult or 0.7–0.9 × 1012 in a 70 kg person.4 If we consider
1
Myeloid Malignancies Section, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA; 2Haematology Research Centre,
Division of Experimental Medicine, Department of Medicine, Imperial College London, London, UK; 3Division of Hematology Products, Office of Hematology and Oncology
Products, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA; 4Division of Hematology, VU University Medical Center,
Amsterdam, The Netherlands; 5Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; 6Department of Medicine, Division of Hematology,
University of Washington, Seattle, WA, USA and 7Department of Epidemiology, University of Washington, Seattle, WA, USA. Correspondence: Dr CS Hourigan, Myeloid
Malignancies Section, Hematology Branch, National Heart, Lung and Blood Institute, National Institutes of Health, Room 10CRC 5-5142, 10 Center Drive, Bethesda, MD 208141476, USA or Dr RB Walter, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, D2-190, Seattle, WA 98109-1024, USA.
E-mail: hourigan@nih.gov or rwalter@fredhutch.org
Received 6 February 2017; revised 15 March 2017; accepted 21 March 2017; accepted article preview online 7 April 2017; advance online publication, 21 April 2017
MRD-Testing in AML
CS Hourigan et al
1483
5% myeloblasts as the cut-off for morphological complete
remission, ~ 1010 leukaemia cells might persist in a person
declared to be in complete remission. Increasing the sensitivity
to detect leukaemia cells below the level of 5 percent was
facilitated by studies in transplantable rat leukaemias5,6 which
reported a linear relationship between survival and numbers of
transferred leukaemia cells.5–7 These data aroused interest in
developing assays to quantify minimal residual disease (MRD) in
humans.8 Although techniques to detect MRD have improved,
current assays can still miss millions of residual leukaemia cells in a
person with AML in complete remission defined by cytomorphology. We therefore believe the abbreviation MRD, first introduced
in 1980(ref. 9) and by now well-established and likely to persist, is
best referred to as measurable residual disease,10 followed by an
expression of the limit of detection and specificity of the MRD-test
being used. Here, we summarize concepts and methods to detect
MRD in persons with AML, evaluate current data, highlight
controversies and suggest future directions (Box 1).
METHODOLOGICAL CONSIDERATIONS OF MRD-TESTING
A perfect MRD-test should accurately identify the smallest
population(s) of leukaemia cells in persons with AML in
morphological complete remission which, if left untreated, cause
relapse while being indifferent towards residual leukaemia cells
that do not cause relapse. For the clinical performance of any
MRD-test, the theoretical maximal sensitivity and specificity of an
assay to detect such residual leukaemia cells, together with other
characteristics (for example, reproducibility), are important, as are
practical considerations regarding sampling details (site, volume,
timing, frequency and so on) and result interpretation, for which
many uncertainties remain.
The clinical utility of MRD tracking in chronic myeloid leukaemia
(CML), using the BCR/ABL1 fusion transcript, is well-established.11–14
This has allowed sensitive quantification of the impact of highly
effective therapy on disease burden and the stratification of patients
based on risk of treatment-failure.15–18 However AML, much more
complex than CML, encompasses a range of myeloid neoplasms
with diverse genetic abnormalities resulting in different histologies, immune phenotypes and clinical outcomes.19–23 Consequently, there is currently no uniform approach to detecting MRD
in persons with AML.
The diverse methods to quantify MRD in AML rely on the either
phenotype or molecular abnormalities of the leukaemia cells.24–30
Multi-parameter flow cytometry (MFC)-based MRD-tests focus on
the phenotype of leukaemia cells. They operate by detecting cell
population(s) which deviate from an antigen-expression pattern
typical of normal or regenerating cells of similar lineage and
maturation stage. Such deviations include cross-lineage expression, over-expression, reduced or absent expression and asynchronous expression.27,30,31 Mutations resulting in gene products
which might be neo-antigens could, in theory, also be identified
by this technique. Advantages of MFC-based MRD-detection
include wide applicability (suitable for almost all persons with
AML if a comprehensive panel of antibodies is used),32–34 ease of
quantifying abnormal cell population(s), relative sensitivity (10 − 3,
that is, 1 in 1000 cells), rapid turn-around and the ability to
distinguish live from dead cells. MFC-based MRD-detection also
allows identification of abnormal cell population(s) with immature
‘stem/progenitor’ phenotype.35,36 However, in addition to limitations common to all MRD-testing discussed below, there are other
limitations inherent to MFC MRD-testing: (1) not all leukaemia cells
have an abnormal phenotype; (2) phenotypes may change over
time with gains/losses of specific abnormalities or patterns of
abnormalities because of disease evolution, sub-clone selection,
and/or progression through the cell cycle;37,38 (3) sensitivity of
MFC-based MRD-testing is less than an optimized PCR-based
MRD-testing (discussed below); (4) MFC-based MRD-detection is
© 2017 Macmillan Publishers Limited, part of Springer Nature.
Box 1
Suggestions for MRD-testing in AML
1. Considerable efforts were, and are, needed to standardize
BCR/ABL1 testing in CML. Given the diverse genetic aetiology
and clonal heterogeneity of AML, even greater standardization efforts are likely needed before MRD-testing is
sufficiently accurate and reproducible to be integrated into
clinical standard-of-care guidelines.
2. No current AML MRD-test has perfect sensitivity and
specificity to accurately predict leukaemia relapse. Physicians
need to carefully consider relapse probability and therapy
risks before proposing an intervention based on results of
MRD-testing.
3. Flow cytometry-based AML MRD-testing is applicable to most
cases but has limitations. AML subtypes caused by, or
associated with, a canonical genetic abnormality (for
example, APL, mutated NPM1 or core-binding factor translocations) may reasonably be monitored using qPCR.
Sequencing-based methods with error correction are likely
to become available soon.
4. We believe some MRD-testing, typically at ⩾ 2 time points,
will be a feature of most high-quality clinical trials in AML in
the future.
5. MRD-testing at one time point may have insufficient
specificity for clinical decision-making whereas trends in
MRD-tests over time are likely to be more informative.
6. Randomized trials (for example, intervention based on MRD
vs no intervention) are required to determine if MRD-guided
therapy is associated with a reduced relapse risk and longer
survival.
7. Determination of end point surrogacy requires multiple
randomized trials to prove the relationship between effect of
treatment on MRD state and the effect of treatment on
survival and/or other clinical benefit end point.
not uniform between people with AML because the ability to
identify abnormal cells depends on the degree residual leukaemia
cells differ from normal cells or from residual leukaemia cells
which do not cause relapse; (5) using MFC appropriately requires
considerable expertise and experience; analysis and data interpretation have some subjective elements and therefore potential
biases (operator-dependent) making assays challenging to harmonize (let alone standardize) across laboratories.39,40 Some, but
not all, of these problems can be reduced with standardized
laboratory procedures including sample processing and instrument settings, single tube approaches with a pre-configured and
stable assay, automated interpretation software, central review
and continuous quality assessment.36,41–43
Quantitative real-time PCR (qPCR) of the chimeric BCR/ABL1
mRNA transcript is a reproducible and highly sensitive technique
to monitor MRD in CML.11,13,44,45 No analogous single canonical
mutation exists in AML, explaining, in part, much of the
enthusiasm for MFC-based MRD-testing despite the limitations
discussed. However, mutations are seen in persons with AML and
substantial work has gone into standardizing qPCR-based assays
for the most common of those that result in chimeric mRNA
transcripts, for example, PML/RARA associated with t(15;17) in
acute promyelocytic leukaemia (APL) and RUNX1/RUNX1T1 (AML1ETO) and CBFB/MYH11 associated with t(8;21) or inv(16) in the
core-binding factor leukaemias.27,46–51 Although these tests are
highly sensitive and threshold levels and/or rates of change
associated with a high probability of relapse can be defined, falsenegative results are still possible. Mutations in nucleophosmin-1
(NPM1) may be tracked effectively using qPCR as well.52–55
Leukemia (2017) 1482 – 1490
MRD-Testing in AML
CS Hourigan et al
1484
Together, these tests cover around 60% of all AML cases in those
under age 60.27 Extensive effort has also gone into developing
MRD-tests targeting detection of transcripts aberrantly expressed
in AML such as Wilms tumour-1 (WT1),56,57 among others.58–61
Increased understanding of the genomic landscape of AML has
prompted considerable interest in developing MRD-tests based on
detecting and quantifying somatic mutations.62,63 This task is
complicated by several factors: (1) there is genetic clonal
heterogeneity at diagnosis with evolution over time and possibly
emergence or selection of small sub-clone(s) at relapse;64–66
(2) error rates intrinsic to most conventional next-generation
sequencing techniques allow only for low-sensitivity detection of
mutated sequences;67 (3) understanding clonality in any sample
may be limited by depth of sequencing and algorithms used for
mutation calling;68 (4) mutated genes (for example, DNMT3A, TET2
and ASXL1 amongst others) can be detected in healthy people
without haematological abnormalities, a condition termed by
some age-related clonal haematopoiesis or clonal haematopoiesis
of indeterminate potential.69–72 This is especially so in older
persons about the same age as most persons with AML; and (5)
some genetic abnormalities persist in persons in long-term
remission, possibly because of residual pre-leukaemia cells or
expansion of normal cells with age-related somatic
mutations.37,66,73–76 Despite these limitations, technical advances
and increasingly sophisticated understanding of the clonal
somatic mutation hierarchy in AML means next-generation
sequencing-based approaches may have an increasing role in
MRD-testing in the future. However, this approach needs to
address the issue that not all mutations have equal biological
consequences in AML and how this computationally demanding
and time-consuming technology can be brought into clinical
practice.
LIMITATIONS OF MRD-TESTING
As intellectually appealing as the concepts underlying MRD tests
in AML may be, there are several practical and logistical
constraints resulting in discordance between theory and
practice.10,27,28,77,78 No current MRD-test has perfect sensitivity
or specificity to accurately predict relapse risk at the cohort level
or, at the more clinically relevant, individual level where therapy
decisions are made. Some persons with a negative MRD-test
relapse (false-negatives) whereas others with a positive MRD-test
do not (false-positives) and are cured, at least operationally. Why is
this so? Besides insufficient sensitivity of the assay, one reason for
false-negative MRD-test results is inconsistent expression of the
designated target(s) or marker(s) of the MRD-test on leukaemia
cells. AML is often an oligo-clonal disease (phenotypically,33
genetically64,79 and in terms of gene expression/epigenetics80)
with clonal selection operating naturally and under pressure from
therapy, such that the target of a previously informative MRD-test
may be less useful at later time points, even in the same person.
Other reasons include inhomogeneous distribution of leukaemia
cells in the bone marrow and/or small sample size(s).3,81
It is a common misperception that technical improvements in
MRD-assays will eventually eliminate false-negative MRD-tests by
providing a complete accounting of the remaining whole-body
disease burden. Rather, the ability to detect low levels of residual
leukaemia cells in AML is limited primarily by the character and
size of the biological sample tested and not MRD-assay
sensitivity.81 Many persons with negative MRD-tests will have
residual leukaemia which may, without additional therapy,
become detectable either by subsequent MRD-testing or by
relapse. This phenomenon is observed in trials of therapy
discontinuation in persons with CML after long-term durable
MRD-negative tests where 50–60 percent of people relapse within
6 months of stopping tyrosine kinase-inhibitor therapy.82–84
Leukemia (2017) 1482 – 1490
The frequency of MRD-testing may also impact test
performance.85 False-positive and false-negative MRD-test results
are more likely with single time point measurements than when
trends in MRD-test results are considered.86 Re-testing can
decrease the likelihood of false-positive (and negative) results.
Sometimes, however, repeat MRD-testing may result in discordance, even in the absence of intervention, which can be bidirectional: a negative-to-positive MRD-test or the converse.
Discordances have many explanations but sampling error is the
dominant issue. Requiring concordant results to declare a person
MRD-positive or -negative increases specificity but decreases
sensitivity.81 Orthogonal validation using alternative methodologies may have utility in such circumstance but may be impractical.
Sequential monitoring can be particularly helpful as a strategy to
increase sensitivity if changes in MRD-levels (for example,
increasing transcript levels) are used as the read-out, and
ordinarily a single discordant datapoint would be insufficient to
make an estimate of clinically relevant changes in residual
leukaemia burden.52,87 The optimal interval and duration of
sequential MRD-testing is unknown and may depend on variables
such as the type of AML85,87 or interval since achieving remission.
Given the potential risk of harm from unnecessary additional
treatment prompted by a false-positive MRD-test some have
advocated for a confirmatory second positive MRD-test within
2–4 weeks of a positive MRD-test before predicting relapse.28
MRD-test results can be falsely positive because of the assay (for
example, technical errors or laboratory contamination) or can fail
to reflect relapse-free clinical outcome because of eradication of
biologically important leukaemia cells with subsequent therapy
(ies) (or, possibly occasionally by immune-mediated anti-leukaemia effects, such as that reported after allotransplants), short
observation interval or early death from causes other than
relapse.81 Important biological reasons to consider for falsepositive MRD-tests are the expression of the MRD marker(s) on
normal cells, pre-leukaemia cells or leukaemia cells unable to
cause relapse.81 Just as the detection of cytogenetic or genetic
abnormalities in an otherwise healthy person is not intrinsically
disease-defining,72,88 detection of some abnormalities targeted by
MRD-testing in a person who has completed therapy does not
necessarily indicate residual AML or relapse risk,73–76,89–91 a
principle more generally seen in oncology.92 There is also the
problem of leukaemia cells resident in unsampled tissues such as
the central nervous system and skin, sites of relapse which could
become more important if MRD-test result-directed therapies are
successful at eliminating bone marrow-based relapses. Lastly,
although results of MRD-testing when done by a laboratory on
stored samples predict clinical outcomes, clearly there is
considerable variation between operators and centres in MRDtesting. This is particularly so for MFC-based MRD-assays,93 which
may be mitigated in part by standardization,94,95 formalized
proficiency testing40 and automated analyses.96 Cross study
comparisons of MRD-testing are limited by the lack of an
independent reference standard for interlaboratory proficiency
testing. Many studies of MRD-testing in AML use the terms MRDnegative and/or -positive, which vary with the sensitivity of the
MRD-test and are impossible to evaluate critically in the absence
of quantitation. Standard laboratory operating procedures including pre-analytical workflow, threshold definitions and reporting
guidelines are used successfully in CML but have not yet been
agreed upon for AML.16,45,97
MRD-TESTING AS TOOL TO PREDICT RELAPSE
Many studies in persons with AML in complete remission,
regardless of the technique used to assess MRD state, report a
robust correlation between a positive MRD-test, a higher risk of
relapse and shorter survivals compared with persons with a
negative MRD-test. These differences are noted as early as 2 weeks
© 2017 Macmillan Publishers Limited, part of Springer Nature.
MRD-Testing in AML
CS Hourigan et al
after beginning induction chemotherapy but are also seen
thereafter, for example, after completing the first or second cycles
of induction chemotherapy, during and after post-remission
chemotherapy and before and after a haematopoietic cell
transplant.49,52,59,61,62,98–114 Comparisons between studies are
complicated by differences in sensitivity, specificity and timing
of MRD-testing and other variables.8,24,26–28,30,115,116 Unsurprisingly, the likelihood of having a positive MRD-test in
cytomorphologically defined complete remission is associated
with the cytogenetic/molecular prognostic-risk category and
with other adverse prognostic factors such as older age,
an antecedent haematologic disorder, prior chemotherapy
and/or radiation therapy or a multiple drug resistance (MDR)
phenotype.112,117–120 Nevertheless, multivariable regression
modelling consistently indicate a positive result of MRD-testing
is an independent prognostic variable for relapse and
survival.52,99–102,104,105,120 An association of MRD-test results and
survival is not directly biologically related but may reflect the
importance of relapse-related deaths on survival rates in many,
but not all, therapy settings. Often the MRD-test result is the most
important factor for relapse and survival in univariate analyses and
is the only prognostic factor in multivariate analyses. Hence,
therapy-response measured by MRD-testing is a stronger predictor of leukaemia relapse than pre-treatment variables and, at
the cohort level, refines risk-stratification beyond that provided by
response assessment by cytomorphology.
Although the prognostic value of MRD-test results in those in
complete remission for predicting subsequent leukaemia relapse
is convincing, the relevance of the rate of change in MRD levels
during therapy is less clear. Studies in children and adults with
AML report persons achieving a complete remission with a
negative MRD-test after the first cycle of induction chemotherapy
have lower cumulative incidences of relapse than those achieving
this state after additional therapy.101,102,104,105 In contrast, in one
study99,100 about 10 percent of subjects with a positive MRD-test
by MFC after induction chemotherapy became MRD-test negative
following post-remission therapy and multivariate analyses
showed an independent association with relapse-free survival
and survival only for post-consolidation but not post-induction
MRD-test results. Similarly, a recent study of the prognostic value
of MRD-testing in children on day 15 after starting induction
chemotherapy and repeated immediately before starting postremission therapy reported a positive MRD-test at the later but not
former time point was an independent prognostic variable for
event-free survival and survival.114 In those followed by RUNX1RUNX1T1 qPCR the most informative landmark for prediction of
relapse and survival outcomes appears to be MRD-test result in
bone marrow samples after completing consolidation
therapy47,50,51 whereas for subjects followed by testing for
mutated NPM1 by qPCR it appears assessing blood samples after
the second cycle of chemotherapy is most accurate.52 This
discordance regarding optimal timing and sample source highlights the need for additional studies—or analyses of data from
from completed studies—to clarify how data from kinetics of
MRD-test result changes during therapy are best used in specific
subtype and therapy contexts to predict clinical outcomes and for
perhaps therapy decision-making in AML. In contrast, for those in
cytomorphological remission after therapy, the rates of increase in
qPCR MRD-test levels associated with relapse are well-defined
with characteristic kinetics in different AML subtypes.49,52,85,86,121
Although current MRD-tests are an important tool for estimating relapse risk in persons with AML in complete remission, a
recent analysis in adults with newly diagnosed de novo AML
treated with intensive chemotherapy on the SWOG S0106 trial
reported MRD-test results on achieving complete remission
improves survival prediction on an subject-level only
minimally.112 Better prediction accuracy is achieved studying
more immediate end points such as 6- and 12-month relapse-free
© 2017 Macmillan Publishers Limited, part of Springer Nature.
survival.112 These data caution against placing too much emphasis
on results of MRD-tests to predict subsequent risk and timing of
relapse at the individual level especially when MRD-testing is done
only at one time point. Features of MRD-testing, which might
affect reproducible correlation with survival in AML, include the
sample tested (blood vs bone marrow, volume), hematopoietic
recovery at time of testing (complete remission vs remission with
incomplete haematopoietic recovery), when during therapy (for
example, after induction vs after post-remission therapy) and how
often (for example, once vs repeated at intervals) the test was
done and other technical parameters.
With many studies claiming results of MRD-testing assessment
of complete remission in AML are better than cytomorphology for
predicting relapse, the question arises as to what extent
conventional assessments of complete remission will continue to
be used in the future. Two studies in children report a poor
correlation between MRD-test results and cytomorphology with a
substantial proportion of subjects with ⩾ 5% myeloblasts but a
negative MRD-test by MFC and/or molecular assays. Others with
o5% myeloblasts had a positive MRD-test.102,103 As in other
studies, a positive MRD-test was strongly associated with relapse
risk whereas myeloblast levels (o 5 vs ⩾ 5%) added no additional
prognostic information. Put otherwise, outcomes of subjects with
a positive MRD-test and o5% myeloblasts and those ⩾ 5%
myeloblasts were similar.103 In addition, in two series of adults
with AML receiving myeloablative allotransplants in complete
remission but with a positive MRD-test had outcomes similar to
persons not in morphological complete remission.59,110,122 These
data suggest results of MRD-testing could supplement or replace
our current cytomorphological based definition of remission.23
Such a change would have significant implications for therapy
algorithms of persons with AML. However, we believe that before
this happens there should be more convincing data that a MRDbased definition of complete remission correlates with increased
survival (or other clinical benefit) in diverse AML populations and
disease state and therapy settings.
MRD-TESTING AS TOOL FOR THERAPY DECISION-MAKING
The close association between MRD-test results and relapse risk
has generated substantial interest in using results of MRD-testing
to direct therapy decisions in persons with AML, for example,
intensify therapy in persons with a positive MRD-test or deintensify therapy in those with a negative MRD-test. Inherent in a
strategy of giving more therapy to someone with a positive MRDtest is the hope the intervention(s) will decrease relapse risk AND
improve survival. Several studies report most persons with AML
and a positive MRD-test relapse within 3–6 months of the
determination. The implication is one would detect relapse by
cytomorphological criteria if one simply waited 3–6 months and
repeated a conventional blood or bone marrow study. Thus,
inherent in the strategy of giving more therapy to someone with a
positive MRD-test is the unproved belief a lead-time of 3–6 months
is important, that is, long-term treatment outcomes will be better
if therapy is given before there is cytomorphological relapse.
The concept of MRD-test-directed therapy has precedent in
persons with acute lymphoblastic leukaemia, where the data from
several non-randomized prospective trials indicate better outcomes with this tactic.123–126 Results of randomized studies are
contradictory. The data from the UKALL 2003 trial in children and
young adults support using results of MRD-testing to direct
therapy-intensity.127,128 In contrast, data from a recent AIEOP-BFM
ALL 2000 trial reported increased relapses when therapy-intensity
was reduced in children with a negative MRD-test.129 In APL,
results of MRD-testing are only available from non-randomized
studies. These data suggest therapy based on results of MRDtesting can prevent clinical relapse.130–132 However, outcomes of
Leukemia (2017) 1482 – 1490
1485
MRD-Testing in AML
CS Hourigan et al
1486
modern therapy of APL are so good that results of MRD-testing
play only a minor role in current clinical practice.
There are no data from randomized studies on the efficacy of
MRD-test directed therapy in non-APL AML. Results from three
prospective, non-randomized multi-centre studies suggest better
outcomes when anti-leukaemia therapy was selected based on
the results of MRD-tests.101,133,134 However, these studies have
important limitations including selection biases, subject and
disease heterogeneity and others which preclude accepting these
data as proof of the value of MRD-testing in directing AML
therapy. There are two additional single centre studies in which
therapy was directed by results of MRD-testing.135,136 Although
both suggest the possibility MRD-directed therapy could decrease
or delay relapse these data should be interpreted cautiously
because the studies were uncontrolled. Even if relapses can be
delayed or avoided in some persons, MRD-directed therapy may
not result in better survival because some persons who relapse
can be rescued with chemotherapy and/or an allotransplant.137
Moreover, pre-emptive therapies based on results of MRD-testing
have toxicities and their impact not only on survival but also
quality-of-life and on the possibility to receive a future transplant
needs careful consideration.
There are many potential biases complicating interpretation of
results of non-randomized clinical trials and rather than concluding these studies prove therapy decisions based on MRD-test data
results in better outcomes in AML, these studies are an impetus to
conduct well-designed, appropriately-controlled trials with sufficient statistical power and observation intervals to evaluate the
potential value of MRD-testing in guiding therapy decisions. There
are many opportunities for the testing of the value, if any, of MRDtesting based therapy-interventions in AML. Value should not be
assumed a priori. Although switching from MRD-test-positivity to
-negativity is a reasonable immediate therapy goal, only
randomized trials can definitively determine whether converting
to MRD-negativity with additional therapy is associated with a
reduced relapse risk and, perhaps, longer survival.
MRD AS SURROGATE END POINT FOR DRUG DEVELOPMENT
AND REGULATORY APPROVAL
From a regulatory perspective, drug approval requires the
demonstration of clinical benefit. Currently, survival is the end
point used as evidence for the clinical benefit of new drugs or new
drug combinations for the therapy of AML. Reduction in AMLassociated symptoms is another potential basis for approval, but
use of this end point is challenging because of the lack of
validated instruments and methodological limitations.138 Obvious
disadvantages of using a survival end point for the purpose of
drug approval include the relatively long duration needed to
complete follow-up, particularly when investigating younger
individuals with AML, and confounding by post-remission or
rescue therapies, for example, transplants.138–140 Use of an early
post-therapy surrogate end point for survival has been suggested
as a way to accelerate AML drug development.138,139
In the regulatory context, a surrogate end point is a marker
thought to predict a clinical outcome such as survival but is not
itself a measure of clinical benefit.141 MRD-test results are an
indirect measure of numbers of residual leukaemia cells and
becoming MRD-test negative may be a plausible indicator of
reduced (or delayed) relapse risk. The question arises whether
becoming MRD-test negative is a biologically plausible surrogate
for improved survival. Perhaps. However, besides biological
plausibility, reliability of a surrogate end point also depends on:
(1) the robustness of the prognostic value of the surrogate for the
clinical outcome; and (2) proof the surrogate quantitatively
captures the effect of therapy on survival or other clinical benefit
end point.142
Leukemia (2017) 1482 – 1490
There has been a long reliance on the use of surrogates to
support drug approvals in oncology (for example, overall response
rates or time-to-progression). In the US, surrogate end points can
be used to support either regular or accelerated approval,
depending on the evidence in support of the surrogate.143
Although MRD-testing could theoretically be used as a surrogate
to support drug approval in AML, surrogacy would first have to be
established.
For regulatory purposes and specifically, the qualification or
acceptance of a surrogate, MRD would need to be proved with
statistical rigour to be a valid surrogate for survival.144,145 The
randomized trials needed to support end point surrogacy differ in
design from those needed to evaluate the value of MRD-test
result-directed therapy. The clinical data needed to support
surrogacy must allow for the determination of the relationship
between the treatment effect on MRD-testing and treatment
effect on the accepted clinical benefit outcome (that is, overall
survival).141,142 The challenge is accumulating a data set large
enough to establish and confirm the optimal parameters of MRDtesting as a correlate of survival, independent of disease state (first
complete remission vs later complete remissions), molecular
subgroup and intensity of induction -or post-remission therapy.
In contrast to cytomorphology-defined complete remission, there
are no convincing analyses showing MRD-defined complete
remission correlates with survival for different AML populations,
for different subtypes of AML, for different disease states or after
different therapies.
Most evaluations of MRD-test results as a surrogate end point in
AML were done in persons receiving induction chemotherapy.
Using MRD-test results to direct therapy, for example, treating
persons in morphological complete remission who switch from a
negative to a positive MRD-test as is done in APL is another area of
interest.146 There are no convincing data that converting from a
positive to negative MRD-test correlates with survival. Until
conversion of MRD-test results is validated as a surrogate
specifically in this setting, randomized trials evaluating the efficacy
of MRD-test result based therapy-interventions need to include a
survival end point or co-end point.
Although some investigators consider event-free survival as
conventionally-defined (cytomorphological relapse or death) a
surrogate for survival in AML, attempts to validate this end point
have been inconsistent.147,148 Whether a positive MRD-test or a
previously negative MRD-test becoming positive should be
considered an event in event-free survival analyses is an
interesting question requiring testing in clinical trials. Although
follow-up of trials using an event-free survival end point might be
briefer compared with a survival end point, a disadvantage is the
need for frequent sampling and testing.143
CONCLUSIONS
Technological advances using diverse techniques have resulted in
MRD-assays with reasonably high sensitivity and specificity. Except
perhaps those AML subtypes caused by or associated with a
canonical genetic abnormality (for example, PML/RARA, mutated
NPM1 or core-binding factor translocations) no single approach to
detect or quantify MRD has been proven superior. Each assay has
advantages and disadvantages needing consideration. Although
MRD-testing is becoming more available, standardization and
harmonization is needed to facilitate comparisons between
studies and for the determination of the value of MRD-testing to
predict relapse, direct therapy and as a potential surrogate end
point for drug approvals. Work toward this goal by the European
LeukemiaNet is ongoing and tools to further this process are
being developed.36,149
Despite the limitations we discuss, many studies report that
results of MRD-testing can inform on relapse risk during and after
AML therapy for cohorts of persons in morphological complete
© 2017 Macmillan Publishers Limited, part of Springer Nature.
MRD-Testing in AML
CS Hourigan et al
1487
remission. For individuals, however, results of testing at a single
landmark time point may only slightly increase the accuracy of
relapse prediction compared to that achieved using current riskstratification approaches.112 The possibility of false-positive and
-negative MRD-tests and of sampling error must also be
considered. Repeat MRD-testing has been shown to improve the
accuracy of relapse prediction and such confirmatory testing may
be an important feature of any clinical use of MRD-testing in
AML.48 Whether and how one should respond to results of the
data from MRD-testing remains to be determined by appropriately
designed clinical trials as it is currently unproven that intervention
will improve survival. An important variable is physician and
patient tolerance for an incorrect MRD-test result and consequences of acting thereon. For example, if therapy prompted by
the MRD-test result has little or no risk of adverse events one
might be willing to accept a false-positive -MRD-test. However, if
the consequences could be dire, tolerance for a false-positive
MRD-test should be less. The treating physician must evaluate
data from cohorts of similar persons, together with an understanding of the limitations of current MRD-testing including
characteristics of the particular test being used, and other personspecific objective and subjective variables, to generate an
integrated relapse risk assessment for the individual they are
evaluating.150
Conventional cytomorphology-based assessment of complete
remission in AML has been repeatedly shown to encompass
widely diverse levels of residual leukaemia cells and to be
associated with a range of clinical outcomes. Consequently, we
believe some estimation of leukaemia state by MRD-testing
(typically at least two informative time points) will likely become
a feature of future AML trials. The small incremental cost increase
of integrating MRD-testing into clinical trials and the lack of a
universally-accepted assay for all AML subtypes need not be
prohibitive. Whether such determinations should be done
routinely outside the context of clinical trials is controversial as
many questions about the optimal use of this information remain.
Although randomized clinical trials evaluating the value of
MRD-testing using different techniques in heterogeneous populations of persons with AML at diverse times during therapy and
across different therapies are clearly needed, data from all clinical
trials could potentially prove useful if carefully annotated with
details of the performance characteristics of the MRD-test used.
The importance of randomized assessments of the value of MRDtesting in AML to predict relapse, direct -therapy and determine if
MRD-test results are a valid surrogate for survival cannot be
overemphasized. If established as a surrogate end point, use of
MRD-testing in AML trials could make drug-testing more time and
cost effective and expedite regulatory approvals. Although there
may be many perceived hurdles for the conduct of such studies,
data from such randomized trials will be critical to determine the
clinical utility of MRD-testing in AML.
CONFLICT OF INTEREST
CSH receives research funding from Merck Sharpe and Dohme and SELLAS Life
Sciences Group AG. RPG is a part-time employee of Celgene Corp. The remaining
authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Frederick R Appelbaum (Fred Hutchinson Cancer Research Center), Anton Hagenbeek
(University of Utrecht), Jacob M Rowe (Sharee Zedek Medical Centre), Charles A
Schiffer (Wayne State University), Jerald P Radich (Fred Hutchinson Cancer Research
Center), Paresh Vyas (University of Oxford) and Donna Przepiorka (US Food and Drug
Administration) kindly reviewed the typescript. This work was supported in part by
the Intramural Research Programs of the National Heart, Lung, and Blood Institute of
the National Institutes of Health. RPG acknowledges support from the National
Institute of Health Research (NIHR) Biomedical Research Centre funding scheme. RBW
is a Leukemia & Lymphoma Society Scholar in Clinical Research. The opinions
© 2017 Macmillan Publishers Limited, part of Springer Nature.
expressed here are ours and do not represent the official position of the National
Institutes of Health, US Food and Drug Administration, or the United States
Government.
REFERENCES
1 Bisel HF. Letter to the editor: criteria for the evaluation of response to treatment
in acute leukemia. Blood 1956; 11: 676–677.
2 Freireich EJ, Gehan EA, Sulman D, Boggs DR, Frei E 3rd. The effect of chemotherapy on acute leukemia in the human. J Chronic Dis 1961; 14: 593–608.
3 Martens ACM, Schultz FW, Hagenbeek A. Nonhomogeneous distribution of
leukemia in the bone marrow during minimal residual disease. Blood 1987; 70:
1073–1078.
4 Harrison WJ. The total cellularity of the bone marrow in man. J Clin Pathol 1962;
15: 254–259.
5 Hagenbeek A, Martens ACM. Minimal residual disease in acute leukaemia: preclinical studies in a relevant rat model (BNML). Baillieres Clin Haematol 1991; 4:
609–635.
6 Hagenbeek A. Minimal residual disease in leukemia: state of the art 1991.
Leukemia 1992: 2: 12–16.
7 Harriss EB, Hoelzer D. Proliferation kinetics of the L 5222 leukaemia in vivo. Leuk
Res 1977; 1: 93–95.
8 Campana D, Pui CH. Detection of minimal residual disease in acute leukemia:
methodologic advances and clinical significance. Blood 1995; 85: 1416–1434.
9 Hagenbeek A, Martens ACM. Kinetics of minimal residual disease in a rat model
for human acute myelocytic leukemia. In: Baum SJ, Ledney GD, van Bekkum DW
(eds). Experimental Hematology Today. Springer: New York, NY, USA, 1980, pp
215–221.
10 Goldman JM, Gale RP. What does MRD in leukemia really mean? Leukemia 2014;
28: 1131.
11 Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes
in chronic myelogenous leukaemia. Nature 1985; 315: 550–554.
12 Hanfstein B, Müller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A et al. Early
molecular and cytogenetic response is predictive for long-term progression-free
and overall survival in chronic myeloid leukemia (CML). Leukemia 2012; 26:
2096–2102.
13 Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF et al.
European LeukemiaNet recommendations for the management of chronic
myeloid leukemia. 2013Blood 2013; 122: 872–884.
14 Hoffmann VS, Baccarani M, Hasford J, Castagnetti F, Di Raimondo F, Casado LF
et al. Treatment and outcome of 2904 CML patients from the EUTOS populationbased registry. Leukemia 2017; 31: 593–601.
15 Paschka P, Müller MC, Merx K, Kreil S, Schoch C, Lahaye T et al. Molecular
monitoring of response to imatinib (Glivec) in CML patients pretreated with
interferon alpha. Low levels of residual disease are associated with continuous
remission. Leukemia 2003; 17: 1687–1694.
16 Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors:
review and recommendations for harmonizing current methodology for
detecting BCR-ABL transcripts and kinase domain mutations and for expressing
results. Blood 2006; 108: 28–37.
17 Hanfstein B, Shlyakhto V, Lauseker M, Hehlmann R, Saussele S, Dietz C et al.
Velocity of early BCR-ABL transcript elimination as an optimized predictor of
outcome in chronic myeloid leukemia (CML) patients in chronic phase on
treatment with imatinib. Leukemia 2014; 28: 1988–1992.
18 Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S et al. Longterm benefits and risks of frontline nilotinib vs imatinib for chronic myeloid
leukemia in chronic phase: 5-year update of the randomized ENESTnd trial.
Leukemia 2016; 30: 1044–1054.
19 Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult
de novo acute myeloid leukemia. N Engl J Med 2013; 368: 2059–2074.
20 Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND et al.
Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med
2016; 374: 2209–2221.
21 Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med
2015; 373: 1136–1152.
22 Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al. The 2016
revision to the World Health Organization classification of myeloid neoplasms
and acute leukemia. Blood 2016; 127: 2391–2405.
23 Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T et al.
Diagnosis and management of AML in adults: 2017 ELN recommendations from
an international expert panel. Blood 2017; 129: 424–447.
24 Buccisano F, Maurillo L, Del Principe MI, Del Poeta G, Sconocchia G, Lo-Coco F
et al. Prognostic and therapeutic implications of minimal residual disease
detection in acute myeloid leukemia. Blood 2012; 119: 332–341.
Leukemia (2017) 1482 – 1490
MRD-Testing in AML
CS Hourigan et al
1488
25 Ravandi F, Jorgensen JL. Monitoring minimal residual disease in acute
myeloid leukemia: ready for prime time? J Natl Compr Canc Netw 2012; 10:
1029–1036.
26 Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat
Rev Clin Oncol 2013; 10: 460–471.
27 Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid
leukemia: which platforms are ready for ‘prime time’? Blood 2014; 124:
3345–3355.
28 Hokland P, Ommen HB, Mulé MP, Hourigan CS. Advancing the minimal residual
disease concept in acute myeloid leukemia. Semin Hematol 2015; 52: 184–192.
29 Duncavage EJ, Tandon B. The utility of next-generation sequencing in diagnosis
and monitoring of acute myeloid leukemia and myelodysplastic syndromes. Int J
Lab Hematol 2015; 37: 115–121.
30 Ommen HB. Monitoring minimal residual disease in acute myeloid leukaemia: a
review of the current evolving strategies. Ther Adv Hematol 2016; 7: 3–16.
31 Wood BL. Principles of minimal residual disease detection for hematopoietic
neoplasms by flow cytometry. Cytometry B Clin Cytom 2016; 90: 47–53.
32 Bahia DM, Yamamoto M, Chauffaille Mde L, Kimura EY, Bordin JO, Filgueiras MA
et al. Aberrant phenotypes in acute myeloid leukemia: a high frequency and its
clinical significance. Haematologica 2001; 86: 801–806.
33 Zelezníková T, Babusíková O. The impact of cell heterogeneity and immunophenotypic changes on monitoring minimal residual disease in acute myeloid
leukemia. Neoplasma 2006; 53: 500–506.
34 Voskova D, Schnittger S, Schoch C, Haferlach T, Kern W. Use of five-color staining
improves the sensitivity of multiparameter flow cytomeric assessment of minimal residual disease in patients with acute myeloid leukemia. Leuk Lymphoma
2007; 48: 80–88.
35 van Rhenen A, Moshaver B, Kelder A, Feller N, Nieuwint AW, Zweegman S et al.
Aberrant marker expression patterns on the CD34+CD38- stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the
normal stem cell compartment both at diagnosis and in remission. Leukemia
2007; 21: 1700–1707.
36 Zeijlemaker W, Kelder A, Oussoren-Brockhoff YJ, Scholten WJ, Snel AN,
Veldhuizen D et al. A simple one-tube assay for immunophenotypical quantification of leukemic stem cells in acute myeloid leukemia. Leukemia 2016; 30:
439–446.
37 Zeijlemaker W, Gratama JW, Schuurhuis GJ. Tumor heterogeneity makes AML a
‘moving target’ for detection of residual disease. Cytometry B Clin Cytom 2014;
86: 3–14.
38 Quesenberry PJ, Goldberg LR, Dooner MS. Concise reviews: a stem cell apostasy:
a tale of four H words. Stem Cells 2015; 33: 15–20.
39 Flanders A, Stetler-Stevenson M, Landgren O. Minimal residual disease testing in
multiple myeloma by flow cytometry: major heterogeneity. Blood 2013; 122:
1088–1089.
40 Keeney M, Halley JG, Rhoads DD, Ansari MQ, Kussick SJ, Karlon WJ et al. Marked
variability in reported minimal residual disease lower level of detection of 4
hematolymphoid neoplasms: a survey of participants in the College of American
Pathologists flow cytometry proficiency testing program. Arch Pathol Lab Med
2015; 139: 1276–1280.
41 Kalina T, Flores-Montero J, van der Velden VHJ, Martin-Ayuso M, Böttcher S,
Ritgen M et al. EuroFlow standardization of flow cytometer instrument settings
and immunophenotyping protocols. Leukemia 2012; 26: 1986–2010.
42 Finak G, Langweiler M, Jaimes M, Malek M, Taghiyar J, Korin Y et al. Standardizing
flow cytometry immunophenotyping analysis from the Human ImmunoPhenotyping Consortium. Sci Rep 2016; 6: 20686.
43 Kalina T, Flores-Montero J, Lecrevisse Q, Pedreira CE, van der Velden VH,
Novakova M et al. Quality assessment program for EuroFlow protocols: summary
results of four-year (2010-2013) quality assurance rounds. Cytometry A 2015; 87:
145–156.
44 Cross NC, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of
molecular response in chronic myeloid leukemia. Leukemia 2012; 26: 2172–2175.
45 Cross NC, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E et al.
Laboratory recommendations for scoring deep molecular responses following
treatment for chronic myeloid leukemia. Leukemia 2015; 29: 999–1003.
46 Gabert J, Beillard E, van der Velden VHJ, Bi W, Grimwade D, Pallisgaard N et al.
Standardization and quality control studies of 'real-time' quantitative reverse
transcriptase polymerase chain reaction of fusion gene transcripts for residual
disease detection in leukemia - a Europe Against Cancer program. Leukemia
2003; 17: 2318–2357.
47 Agrawal M, Corbacioglu A, Paschka P, Weber D, Gaidzik VI, Jahn N et al. Minimal
residual disease monitoring in acute myeloid leukemia (AML) with translocation
t(8;21)(q22;q22): results of the AML Study Group (AMLSG). Blood 2016; 128:
1207–1207.
48 Willekens C, Blanchet O, Renneville A, Cornillet-Lefebvre P, Pautas C, Guieze R
et al. Prospective long-term minimal residual disease monitoring using RQ-PCR
Leukemia (2017) 1482 – 1490
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
in RUNX1-RUNX1T1-positive acute myeloid leukemia: results of the French
CBF-2006 trial. Haematologica 2016; 101: 328–335.
Yin JA, O'Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual
disease monitoring by quantitative RT-PCR in core binding factor AML allows risk
stratification and predicts relapse: results of the United Kingdom MRC
AML-15 trial. Blood 2012; 120: 2826–2835.
Weisser M, Haferlach C, Hiddemann W, Schnittger S. The quality of molecular
response to chemotherapy is predictive for the outcome of AML1-ETO-positive
AML and is independent of pretreatment risk factors. Leukemia 2007; 21:
1177–1182.
Schnittger S, Weisser M, Schoch C, Hiddemann W, Haferlach T, Kern W. New
score predicting for prognosis in PML-RARA+, AML1-ETO+, or CBFBMYH11+
acute myeloid leukemia based on quantification of fusion transcripts. Blood
2003; 102: 2746–2755.
Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A et al. Assessment of
minimal residual disease in standard-risk AML. N Engl J Med 2016; 374: 422–433.
Gorello P, Cazzaniga G, Alberti F, Dell'Oro MG, Gottardi E, Specchia G et al.
Quantitative assessment of minimal residual disease in acute myeloid leukemia
carrying nucleophosmin (NPM1) gene mutations. Leukemia 2006; 20: 1103–1108.
Krönke J, Schlenk RF, Jensen KO, Tschürtz F, Corbacioglu A, Gaidzik VI et al.
Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group.
J Clin Oncol 2011; 29: 2709–2716.
Shayegi N, Kramer M, Bornhäuser M, Schaich M, Schetelig J, Platzbecker U et al.
The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood 2013; 122: 83–92.
Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV et al. Real-time
quantitative polymerase chain reaction detection of minimal residual disease by
standardized WT1 assay to enhance risk stratification in acute myeloid leukemia:
a European LeukemiaNet study. J Clin Oncol 2009; 27: 5195–5201.
Nomdedéu JF, Hoyos M, Carricondo M, Bussaglia E, Estivill C, Esteve J et al. Bone
marrow WT1 levels at diagnosis, post-induction and post-intensification in adult
de novo AML. Leukemia 2013; 27: 2157–2164.
Steinbach D, Schramm A, Eggert A, Onda M, Dawczynski K, Rump A et al.
Identification of a set of seven genes for the monitoring of minimal residual
disease in pediatric acute myeloid leukemia. Clin Cancer Res 2006; 12:
2434–2441.
Goswami M, McGowan KS, Lu K, Jain N, Candia J, Hensel NF et al. A multigene
array for measurable residual disease detection in AML patients undergoing SCT.
Bone Marrow Transplant 2015; 50: 642–651.
Steinbach D, Bader P, Willasch A, Bartholomae S, Debatin KM, Zimmermann M
et al. Prospective validation of a new method of monitoring minimal residual
disease in childhood acute myelogenous leukemia. Clin Cancer Res 2015; 21:
1353–1359.
Mulé MP, Mannis GN, Wood BL, Radich JP, Hwang J, Ramos NR et al. Multigene
measurable residual disease assessment improves acute myeloid leukemia
relapse risk stratification in autologous hematopoietic cell transplantation. Biol
Blood Marrow Transplant 2016; 22: 1974–1982.
Klco JM, Miller CA, Griffith M, Petti A, Spencer DH, Ketkar-Kulkarni S et al.
Association between mutation clearance after induction therapy and outcomes
in acute myeloid leukemia. JAMA 2015; 314: 811–822.
Uy GL, Duncavage EJ, Chang GS, Jacoby MA, Miller CA, Shao J et al. Dynamic
changes in the clonal structure of MDS and AML in response to epigenetic
therapy. Leukemia 2016; 31: 872–881.
Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS et al. Clonal evolution
in relapsed acute myeloid leukaemia revealed by whole-genome sequencing.
Nature 2012; 481: 506–510.
Klco JM, Spencer DH, Miller CA, Griffith M, Lamprecht TL, O'Laughlin M et al.
Functional heterogeneity of genetically defined subclones in acute myeloid
leukemia. Cancer Cell 2014; 25: 379–392.
Grimwade D, Ivey A, Huntly BJ. Molecular landscape of acute myeloid leukemia
in younger adults and its clinical relevance. Blood 2016; 127: 29–41.
Young AL, Wong TN, Hughes AE, Heath SE, Ley TJ, Link DC et al. Quantifying
ultra-rare pre-leukemic clones via targeted error-corrected sequencing. Leukemia
2015; 29: 1608–1611.
Griffith M, Miller CA, Griffith OL, Krysiak K, Skidmore ZL, Ramu A et al. Optimizing
cancer genome sequencing and analysis. Cell Syst 2015; 1: 210–223.
Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF et al.
Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence.
N Engl J Med 2014; 371: 2477–2487.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG et al. Agerelated clonal hematopoiesis associated with adverse outcomes. N Engl J Med
2014; 371: 2488–2498.
© 2017 Macmillan Publishers Limited, part of Springer Nature.
MRD-Testing in AML
CS Hourigan et al
1489
71 Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP et al.
Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015; 126: 9–16.
72 Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun
2016; 7: 12484.
73 Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing nonleukemic stem
cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc
Natl Acad Sci USA 2000; 97: 7521–7526.
74 Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci USA 2014; 111: 2548–2553.
75 Pløen GG, Nederby L, Guldberg P, Hansen M, Ebbesen LH, Jensen UB et al.
Persistence of DNMT3A mutations at long-term remission in adult patients
with AML. Br J Haematol 2014; 167: 478–486.
76 Wong TN, Miller CA, Klco JM, Petti A, Demeter R, Helton NM et al. Rapid
expansion of preexisting nonleukemic hematopoietic clones frequently follows
induction therapy for de novo AML. Blood 2016; 127: 893–897.
77 Gale RP. Measureable residual disease (MRD): much ado about nothing? Bone
Marrow Transplant 2015; 50: 163–164.
78 Prasad V, Gale RP. Precision medicine in acute myeloid leukemia: hope, hype
or both? Leuk Res 2016; 48: 73–77.
79 Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC et al. The origin and
evolution of mutations in acute myeloid leukemia. Cell 2012; 150: 264–278.
80 Li S, Garrett-Bakelman FE, Chung SS, Sanders MA, Hricik T, Rapaport F et al.
Distinct evolution and dynamics of epigenetic and genetic heterogeneity in
acute myeloid leukemia. Nat Med 2016; 22: 792–799.
81 Butturini A, Klein J, Gale RP. Modeling minimal residual disease (MRD)-testing.
Leuk Res 2003; 27: 293–300.
82 Deininger MW. Molecular monitoring in CML and the prospects for treatmentfree remissions. Hematology Am Soc Hematol Educ Program 2015; 2015: 257–263.
83 Saußele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free
remission in chronic myeloid leukemia. Leukemia 2016; 30: 1638–1647.
84 Rea D, Nicolini FE, Tulliez M, Guilhot F, Guilhot J, Guerci-Bresler A et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim
analysis of the STOP 2G-TKI study. Blood 2017; 129: 846–854.
85 Ommen HB, Schnittger S, Jovanovic JV, Ommen IB, Hasle H, Østergaard M et al.
Strikingly different molecular relapse kinetics in NPM1c, PML-RARA, RUNX1RUNX1T1, and CBFB-MYH11 acute myeloid leukemias. Blood 2010; 115: 198–205.
86 Hokland P, Ommen HB. Towards individualized follow-up in adult acute myeloid
leukemia in remission. Blood 2011; 117: 2577–2584.
87 Hills RK, Ivey A, Grimwade D, Group UKNCRIAW. Assessment of minimal residual
disease in standard-risk AML. N Engl J Med 2016; 375: e9.
88 Song J, Mercer D, Hu X, Liu H, Li MM. Common leukemia- and lymphomaassociated genetic aberrations in healthy individuals. J Mol Diagn 2011; 13:
213–219.
89 Tobal K, Liu Yin JA. RT-PCR method with increased sensitivity shows persistence
of PML-RARA fusion transcripts in patients in long-term remission of APL.
Leukemia 1998; 12: 1349–1354.
90 Showel MM, Brodsky RA, Tsai HL, Briel KM, Kowalski J, Griffin CA et al. Isolated
clonal cytogenetic abnormalities after high-dose therapy. Biol Blood Marrow
Transplant 2014; 20: 1130–1138.
91 Farina M, Rossi G, Bellotti D, Marchina E, Gale RP. Is having clonal cytogenetic
abnormalities the same as having leukaemia. Acta Haematol 2016; 135: 39–42.
92 Bäsecke J, Griesinger F, Trümper L, Brittinger G. Leukemia- and lymphomaassociated genetic aberrations in healthy individuals. Ann Hematol 2002; 81:
64–75.
93 Buckley SA, Wood BL, Othus M, Hourigan CS, Ustun C, Linden MA et al. Minimal
residual disease prior to allogeneic hematopoietic cell transplantation in acute
myeloid leukemia: a meta-analysis. Haematologica 2017; e-pub ahead of print
25 January 2017; doi:10.3324/haematol.2016.159343.
94 van de Loosdrecht AA, Alhan C, Béné MC, Della Porta MG, Dräger AM, Feuillard J
et al. Standardization of flow cytometry in myelodysplastic syndromes: report
from the first European LeukemiaNet working conference on flow cytometry in
myelodysplastic syndromes. Haematologica 2009; 94: 1124–1134.
95 Zini G, Kern W, Brereton M, Stephens AD. ICSH: on board for new projects. Int J
Lab Hematol 2014; 36: 306–312.
96 Ni W, Hu B, Zheng C, Tong Y, Wang L, Li QQ et al. Automated analysis of acute
myeloid leukemia minimal residual disease using a support vector machine.
Oncotarget 2016; 7: 71915–71921.
97 Foroni L, Wilson G, Gerrard G, Mason J, Grimwade D, White HE et al. Guidelines
for the measurement of BCR-ABL1 transcripts in chronic myeloid leukaemia. Br J
Haematol 2011; 153: 179–190.
98 Kern W, Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T. Prognostic impact of early response to induction therapy as assessed by
© 2017 Macmillan Publishers Limited, part of Springer Nature.
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
multiparameter flow cytometry in acute myeloid leukemia. Haematologica 2004;
89: 528–540.
Maurillo L, Buccisano F, Del Principe MI, Del Poeta G, Spagnoli A, Panetta P et al.
Toward optimization of postremission therapy for residual disease-positive
patients with acute myeloid leukemia. J Clin Oncol 2008; 26: 4944–4951.
Buccisano F, Maurillo L, Spagnoli A, Del Principe MI, Fraboni D, Panetta P et al.
Cytogenetic and molecular diagnostic characterization combined to postconsolidation minimal residual disease assessment by flow cytometry improves
risk stratification in adult acute myeloid leukemia. Blood 2010; 116: 2295–2303.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J et al. Minimal
residual disease-directed therapy for childhood acute myeloid leukaemia: results
of the AML02 multicentre trial. Lancet Oncol 2010; 11: 543–552.
Loken MR, Alonzo TA, Pardo L, Gerbing RB, Raimondi SC, Hirsch BA et al. Residual
disease detected by multidimensional flow cytometry signifies high relapse risk
in patients with de novo acute myeloid leukemia: a report from Children's
Oncology Group. Blood 2012; 120: 1581–1588.
Inaba H, Coustan-Smith E, Cao X, Pounds SB, Shurtleff SA, Wang KY et al.
Comparative analysis of different approaches to measure treatment response in
acute myeloid leukemia. J Clin Oncol 2012; 30: 3625–3632.
Freeman SD, Virgo P, Couzens S, Grimwade D, Russell N, Hills RK et al. Prognostic
relevance of treatment response measured by flow cytometric residual disease
detection in older patients with acute myeloid leukemia. J Clin Oncol 2013; 31:
4123–4131.
Terwijn M, van Putten WL, Kelder A, van der Velden VH, Brooimans RA, Pabst T
et al. High prognostic impact of flow cytometric minimal residual disease
detection in acute myeloid leukemia: data from the HOVON/SAKK AML
42A study. J Clin Oncol 2013; 31: 3889–3897.
Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P et al.
Prospective evaluation of gene mutations and minimal residual disease in
patients with core binding factor acute myeloid leukemia. Blood 2013; 121:
2213–2223.
Marani C, Clavio M, Grasso R, Colombo N, Guolo F, Kunkl A et al. Integrating post
induction WT1 quantification and flow-cytometry results improves minimal
residual disease stratification in acute myeloid leukemia. Leuk Res 2013; 37:
1606–1611.
Chen X, Xie H, Wood BL, Walter RB, Pagel JM, Becker PS et al. Relation of clinical
response and minimal residual disease and their prognostic impact on outcome
in acute myeloid leukemia. J Clin Oncol 2015; 33: 1258–1264.
Chen X, Xie H, Estey EH. Reply to D. Przepiorka et al. J Clin Oncol 2015; 33:
3676–3677.
Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y et al. Allogeneic
hematopoietic cell transplantation for acute myeloid leukemia: time to move
toward a minimal residual disease-based definition of complete remission? J Clin
Oncol 2016; 34: 329–336.
Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB et al. Pre- and posttransplant quantification of measurable ('minimal') residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia 2016; 30:
1456–1464.
Othus M, Wood BL, Stirewalt DL, Estey EH, Petersdorf SH, Appelbaum FR et al.
Effect of measurable ('minimal') residual disease (MRD) information on prediction of relapse and survival in adult acute myeloid leukemia. Leukemia 2016; 30:
2080–2083.
Vidriales MB, Pérez-López E, Pegenaute C, Castellanos M, Pérez JJ, Chandía M
et al. Minimal residual disease evaluation by flow cytometry is a complementary
tool to cytogenetics for treatment decisions in acute myeloid leukaemia. Leuk
Res 2016; 40: 1–9.
Tierens A, Bjørklund E, Siitonen S, Marquart HV, Wulff-Juergensen G, Pelliniemi TT
et al. Residual disease detected by flow cytometry is an independent predictor
of survival in childhood acute myeloid leukaemia; results of the NOPHO-AML
2004 study. Br J Haematol 2016; 174: 600–609.
Campana D, Leung W. Clinical significance of minimal residual disease in
patients with acute leukaemia undergoing haematopoietic stem cell transplantation. Br J Haematol 2013; 162: 147–161.
Buckley SA, Appelbaum FR, Walter RB. Prognostic and therapeutic implications
of minimal residual disease at the time of transplantation in acute leukemia.
Bone Marrow Transplant 2013; 48: 630–641.
San Miguel JF, Martinez A, Macedo A, Vidriales MB, Lopez-Berges C, Gonzalez M
et al. Immunophenotyping investigation of minimal residual disease is a useful
approach for predicting relapse in acute myeloid leukemia patients. Blood 1997;
90: 2465–2470.
Venditti A, Buccisano F, Del Poeta G, Maurillo L, Tamburini A, Cox C et al. Level of
minimal residual disease after consolidation therapy predicts outcome in acute
myeloid leukemia. Blood 2000; 96: 3948–3952.
Buccisano F, Maurillo L, Gattei V, Del Poeta G, Del Principe MI, Cox MC et al. The
kinetics of reduction of minimal residual disease impacts on duration of
Leukemia (2017) 1482 – 1490
MRD-Testing in AML
CS Hourigan et al
1490
120
121
122
123
124
125
126
127
128
129
130
131
132
133
134
response and survival of patients with acute myeloid leukemia. Leukemia 2006;
20: 1783–1789.
Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML et al. Impact of
pretransplantation minimal residual disease, as detected by multiparametric
flow cytometry, on outcome of myeloablative hematopoietic cell transplantation
for acute myeloid leukemia. J Clin Oncol 2011; 29: 1190–1197.
Ommen HB, Hokland P, Haferlach T, Abildgaard L, Alpermann T, Haferlach C et al.
Relapse kinetics in acute myeloid leukaemias with MLL translocations or partial
tandem duplications within the MLL gene. Br J Haematol 2014; 165: 618–628.
Hourigan CS, Goswami M, Battiwalla M, Barrett AJ, Sheela S, Karp JE et al. When
the minimal becomes measurable. J Clin Oncol 2016; 34: 2557–2558.
Yeoh AE, Ariffin H, Chai EL, Kwok CS, Chan YH, Ponnudurai K et al. Minimal
residual disease-guided treatment deintensification for children with acute
lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol 2012; 30: 2384–2392.
Eckert C, Henze G, Seeger K, Hagedorn N, Mann G, Panzer-Grümayer R et al. Use of
allogeneic hematopoietic stem-cell transplantation based on minimal residual
disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol 2013; 31: 2736–2742.
Roberts KG, Pei D, Campana D, Payne-Turner D, Li Y, Cheng C et al. Outcomes of
children with BCR-ABL1-like acute lymphoblastic leukemia treated with riskdirected therapy based on the levels of minimal residual disease. J Clin Oncol
2014; 32: 3012–3020.
Pui CH, Pei D, Coustan-Smith E, Jeha S, Cheng C, Bowman WP et al. Clinical utility
of sequential minimal residual disease measurements in the context of riskbased therapy in childhood acute lymphoblastic leukaemia: a prospective study.
Lancet Oncol 2015; 16: 465–474.
Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R et al. Treatment
reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised
controlled trial. Lancet Oncol 2013; 14: 199–209.
Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C et al. Augmented
post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and
intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised
controlled trial. Lancet Oncol 2014; 15: 809–818.
Schrappe M, Zimmermann M, Möricke A, Mann G, Valsecchi MG, Bartram CT
et al. Reduced intensity delayed intensification in standard-risk patients defined
by minimal residual disease in childhood acute lymphoblastic leukemia: results
of an international randomized trial in 1164 patients (trial AIEOP-BFM ALL 2000)
[abstract]. Blood 2016; 126: 4.
Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM et al. Therapy of
molecular relapse in acute promyelocytic leukemia. Blood 1999; 94: 2225–2229.
Esteve J, Escoda L, Martín G, Rubio V, Díaz-Mediavilla J, González M et al. Outcome of patients with acute promyelocytic leukemia failing to front-line treatment with all-trans retinoic acid and anthracycline-based chemotherapy
(PETHEMA protocols LPA96 and LPA99): benefit of an early intervention.
Leukemia 2007; 21: 446–452.
Grimwade D, Jovanovic JV, Hills RK. Can we say farewell to monitoring minimal
residual disease in acute promyelocytic leukaemia? Best Pract Res Clin Haematol
2014; 27: 53–61.
Zhu HH, Zhang XH, Qin YZ, Liu DH, Jiang H, Chen H et al. MRD-directed risk
stratification treatment may improve outcomes of t(8;21) AML in the first
complete remission: results from the AML05 multicenter trial. Blood 2013; 121:
4056–4062.
Balsat M, Renneville A, Thomas X, de Botton S, Caillot D, Marceau A et al.
Postinduction minimal residual disease predicts outcome and benefit from
allogeneic stem cell transplantation in acute myeloid leukemia with NPM1
mutation: a study be the Acute Leukemia French Association Group. J Clin Oncol
2017; 35: 185–193.
Leukemia (2017) 1482 – 1490
135 Sockel K, Wermke M, Radke J, Kiani A, Schaich M, Bornhäuser M et al. Minimal
residual disease-directed preemptive treatment with azacitidine in patients with
NPM1-mutant acute myeloid leukemia and molecular relapse. Haematologica
2011; 96: 1568–1570.
136 Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A et al.
Azacitidine for treatment of imminent relapse in MDS or AML patients after
allogeneic HSCT: results of the RELAZA trial. Leukemia 2012; 26: 381–389.
137 Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J et al. Curability of
patients with acute myeloid leukemia who did not undergo transplantation in
first remission. J Clin Oncol 2013; 31: 1293–1301.
138 Appelbaum FR, Rosenblum D, Arceci RJ, Carroll WL, Breitfeld PP, Forman SJ et al.
End points to establish the efficacy of new agents in the treatment of acute
leukemia. Blood 2007; 109: 1810–1816.
139 Walter RB, Appelbaum FR, Tallman MS, Weiss NS, Larson RA, Estey EH. Shortcomings in the clinical evaluation of new drugs: acute myeloid leukemia as
paradigm. Blood 2010; 116: 2420–2428.
140 Estey E, Othus M, Lee SJ, Appelbaum FR, Gale RP. New drug approvals in acute
myeloid leukemia: what's the best end point? Leukemia 2016; 30: 521–525.
141 US Department of Health and Human Services, Food and Drug AdministrationGuidance for Industry: Expedited Programs for Serious Conditions - Drugs
and Biologics (2014). Available at: http://www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm358301.pdf, pp17-18 (accessed
15 November 2016).
142 US Department of Health and Human Services, Food and Drug AdministrationGuidance for Industry: E9 Statistical Principles for Clinical Trials (1998).
Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulator
yinformation/guidances/ucm073137.pdf, p. 9 (accessed 9 September 2016).
143 US Department of Health and Human Services, Food and Drug AdministrationGuidance for Industry: Clinical Trial Endpoints for the Approval of Cancer
Drugs and Biologics (2007). Available at: http://www.fda.gov/downloads/
Drugs/.../Guidances/ucm071590.pdf, pp. 8-9 (accessed 9 September 2016).
144 Buyse M, Burzykowski T, Carroll K, Michiels S, Sargent DJ, Miller LL et al.
Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol 2007; 25: 5218–5224.
145 Sargent DJ, Qian S, De Bedout S, Flowers C, Fowler NH, Fu T et al. Evaluation of
complete response rate at 30 months (CR30) as a surrogate for progression-free
survival (PFS) in first-line follicular lymphoma (FL) studies: results from the
prospectively specified Follicular Lymphoma Analysis of Surrogacy Hypothesis
(FLASH) analysis with individual patient data (IPD) of 3,837 patients (pts)
[abstract]. J Clin Oncol 2015; 33: 8504.
146 Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of
chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemiafree survival. J Clin Oncol 2011; 29: 2493–2498.
147 Schlenk RF, Döhner H, Döhner K, Ganser A, Heuser M, Benner A et al. Event-free
survival is a surrogate for overall survival in patients treated for acute myeloid
leukemia [abstract]. Blood 2015; 126: 3744.
148 Othus M, van Putten W, Löwenberg B, Petersdorf SH, Nand S, Erba H et al.
Relationship between event-free survival and overall survival in acute myeloid
leukemia: a report from SWOG, HOVON/SAKK, and MRC/NCRI. Haematologica
2016; 101: e284–e286.
149 Østergaard M, Nyvold CG, Jovanovic JV, Andersen MT, Kairisto V, Morgan YG
et al. Development of standardized approaches to reporting of minimal residual
disease data using a reporting software package designed within the European
LeukemiaNet. Leukemia 2011; 25: 1168–1173.
150 Sniderman AD, D'Agostino RB Sr, Pencina MJ. The role of physicians in the era of
predictive analytics. JAMA 2015; 314: 25–26.
© 2017 Macmillan Publishers Limited, part of Springer Nature.