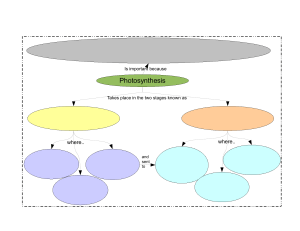
Dissolved Oxygen The Good Gas Dissolved oxygen (DO) Dissolved oxygen (DO) is the amount of oxygen that is present in the water. It is measured in milligrams per liter (mg/L), or the number of milligrams of oxygen dissolved in a liter of water. How does oxygen get into the water? Oxygen gets into the water when: ➢Oxygen from the atmosphere dissolves and mixes into the water’s surface ➢Algae and bay grasses release oxygen during photosynthesis ➢Water flows into the Bay from stream, river and the ocean.Ocean waters generally have more oxygen. River waters are fast-moving, which helps oxygen from the air mix in. Photosynthesis: Your one-stop shop for all of your oxygen needs! Solar energy + 6CO2 + 6H2O → C6H12O6 + 6O2 Happy Rays of Sunshine Carbon Dioxide (from air) Oxygen Carbohydrate (plant material) (to air) Water (from ground) Aquatic plants and phytoplankton (single cell floating plants) release oxygen into the water as a product of photosynthesis Happy Rays of Sunshine CO2 O2 Oxygen: A Soluble Gas H 2O O2 H2O H O 2 O O2 2 O2 H 2O O2 H O H O 2 2 O2 H2O O2 H O H O 2 2 H2O Habitat Classification Based on DO Concentration DO Concentration (mg/L) 3 2.5 > 2 mg/L Normoxic 2 1.5 1 0.2 – 2 mg/L Hypoxic 0 – 0.2 mg/L Anoxic 0.5 0 Most fish need oxygen levels > 2.0 mg/L 0-2 mg/L: not enough oxygen to support life. 2-4 mg/L: only a few fish and aquatic insects can survive. 4-7 mg/L: good for many aquatic animals, low for cold water fish 7-11 mg/L: very good for most stream fish Daily Aquatic Oxygen Cycle Dissolved oxygen reaches its peak during the day. At night, it decreases as photosynthesis has stopped while oxygen consuming processes such as respiration, oxidation, and respiration continue, until shortly before dawn. Daily Aquatic Oxygen Cycle Moonshine Sunshine Moonshine Dissolved Oxygen 4 3 2 1 0 Midnight Sunrise Noon Sunset Midnight Dissolved oxygen reaches its peak during the day. At night, it decreases as photosynthesis has stopped while oxygen consuming processes such as respiration, oxidation, and respiration continue, until shortly before dawn. Decomposition – Not good for DO • Decomposer organisms (mainly bacteria) consume oxygen – Sometimes consume oxygen faster than plants can produce it, even during the middle of the day! • A sudden increase in organic matter (think leaf litter) can create a spike in decomposition activity – especially if it is hot – Hurricanes not only add organic matter to our waterways, but also stir up the sediment. – Can cause fish kills!! Factors Affecting DO ➢Volume and velocity of water flowing in the water body ➢Climate/Season ➢The type and number of organisms in the water body ➢Altitude ➢Dissolved or suspended solids ➢Amount of nutrients in the water ➢Organic Wastes ➢Riparian Vegetation ➢Groundwater Inflow Wind Stirs in atmospheric oxygen Current Velocity • The faster water flows, the more atmospheric oxygen is mixed into the water. Water Clarity Amount of Sunlight Reaching Plants The muddier the water is, the less light reaches the plants! Cloud Cover • Clouds decrease the amount of sunlight reaching aquatic plants, thus oxygen production is reduced. Human factors that affect dissolved oxygen in streams include : ➢ addition of oxygen consuming organic wastes such as sewage, ➢ addition of nutrients, ➢ changing the flow of water, ➢ raising the water temperature, and ➢ the addition of chemicals. Sample Collection ➢ Remember that the water sample must be collected in such a way that you can cap the bottle while it is still submerged. ➢ Carefully wade into the stream. Collect the sample so that you are not standing upstream of the bottle. ➢ Remove the cap of the BOD bottle. Slowly lower the bottle into the water, pointing it downstream, until the lower lip of the opening is just submerged. ➢ Cap the bottle while it is still submerged. Lift it out of the water and look around the "collar" of the bottle just below the bottom of the stopper. If you see an air bubble, pour out the sample and try again. ➢ "Fix" the sample immediately following the directions in your kit. SAMPLE HANDLING AND PRESERVATION Preservation of sample is not practical. Because biological activity will continue after a sample has been taken, changes may occur during handling and storage. If analysis is to be carried out within two hours of collection, cool storage is not necessary. If analysis cannot be started with in the two hours of sample collection to reduce the change in sample keep all samples at 4°C. Do not allow samples to freeze. Analysis should begin as shown as possible. Do not open sample bottle before analysis. Begin analysis within six hours of sample collection.