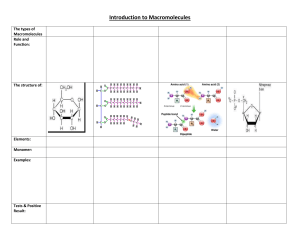
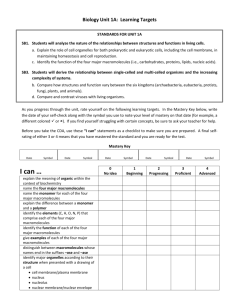
Reginald H. Garrett Charles M. Grisham www.cengage.com/chemistry/garrett CHAPTER 1 Chemistry is the Logic of Biological Phenomena Dr. J.K. Lu 2-18-2014 Reginald Garrett & Charles Grisham • University of Virginia Outline 1.1 1.2 1.3 1.4 1.5 1.6 On Life and Chemistry... Distinctive Properties of Living Systems Biomolecules: Molecules of Life Biomolecular Hierarchy Properties of Biomolecules Organization and Structure of Cells Viruses as Cell Parasites “Living things are composed of lifeless molecules” (Albert Lehninger) “Chemistry is the logic of biological phenomena” (Garrett and Grisham) Distinctive Properties of Living Systems Life a complex phenomena, impossible to define in a precise fashion Life possesses the properties of replication, catalysis, and mutability - Norman Horowitz Organisms are complicated and highly organized Biological structures serve functional purposes Living systems are actively engaged in energy transformations Living systems have a remarkable capacity for selfreplication Living organisms objective 1: Complexity & Organization Living Organisms Objective 2: Nutrient required 2 Cellular Architecture 1.1 – What Are the Distinctive Properties of Living Systems? Solar energy flows from photosynthetic organisms through food chains to herbivores and on to carnivores at the apex of the food pyramid. Food pyramid Figure 1.2 The food pyramid. Photosynthetic organisms at the base capture light energy. Herbivores and carnivores derive their energy ultimately from these primary producers. Two biochemical important energyrich compound Living Organisms Objective 3: Biological reproduction The Fidelity of Self-Replication Resides Ultimately in the Chemical Nature of DNA Diverse living organisms share common chemical features Figure 1.5 The DNA double helix. Two complementary polynucleotide chains running in opposite directions can pair through hydrogen bonding between their nitrogenous bases. Their complementary nucleotide sequences give rise to structural complementarity. BIOCHEMISTRY Bioechemistry is… A study of the molecular basis of life “The study of life on the molecular level” - D. Voet & J. Voet (NOTE: Why YOU need to study “ Biochemistry” ?!) Goals of Biochemistry Describe structure, organization, function of cells in molecular terms: - Structural Chemistry - Metabolism - Molecular Genetics Chemical mechanisms of many central processes of life now understood common molecular patterns and principles biochemistry is profoundly influencing medicine Advanced molecular biology, biotechnology Biochemistry at Revolution in biological sciences e.g. Designing Molecules (medical applications) 6-Mercaptopurine 3'-Azido-2',3'-dideoxythymidine (AZT) Isoproterenol What property unites H, O, C and N and renders these atoms so appropriate to the chemistry of life? As: Their ability to form covalent bonds by electron-pair sharing Biomoecules Elements: hydrogen, oxygen, carbon, nitrogen(lightest elements of the periodic table capable of forming a variety of strong covalent bonds Compounds: carbon-based compounds 1.2 Biomolecules:The Molecules of Life What are the bond energies of covalent bonds? Bond H-H C-H C-C C-O Example of the versatility of C-C bonds in building complex structures Energy kJ/mol 436 414 343 351 - essential metals: Na+, K+, Mg+2, Ca+2, ClFig. 1-7, p. 6 Table 1-4, p. 13 Fig. 1-14, p. 13 1.2 Biomolecules: The Molecules of Life H, O, C and N make up 99+% of atoms in the human body ELEMENT Oxygen Hydrogen Carbon Nitrogen PERCENTAGE 63 25.2 9.5 1.4 Table 1-2, p. 7 Elements found In organisms Biomolecular hiearchchy Fig1-36 The three stages of the evolution of life Page 30 Page 32 Fig 1-37 Apparatus for emulating the synthesis of organic compounds on the prebiotic Earth Simple compounds: H2O, CO2, NH4+, NO3-, N2 Metabolites Building blocks: amino acids, nucleiotide, monosaccharides Macromolecules: protein,nucleic acids, polysaccharides 1.3 A Biomolecular Hierarchy Simple Molecules are the Units for Building Complex Structures Molecular organization In the cell is hierarchy Metabolites and Macromolecules Organelles Membranes The Unit of Life is the Cell 1.4 Properties of Biomolecules Reflect Their Fitness to the Living Condition Macromolecules and Their Building Blocks Have a “Sense” or Directionality Macromolecules are Informational Biomolecules have Characteristic ThreeDimensional Architecture Weak Forces Maintain Biological Structure and Determine Biomolecular Interactions Fig. 1-9a, p. 10 Fig. 1-9b, p. 10 Fig. 1-9c, p. 10 Biological Molecules: Macromolecules The molecular basis of life Monomers/Polymers (Figure 1.7) v Sugar/Polysaccharide v Nucleotide/Nucleic Acids v Amino acid/Polypeptides (Figure 1.6) Revolves around the organization and interrelationship between the 4 building blocks of life Fig. 1-9, p. 10 1.4 Properties of Biomolecules Reflect Their Fitness to the Living Condition 3-Dimensional architecture and intermolecular interactions (via complementary surfaces of macromolecules are based on weak forces Information content: sequence of monomer building blocks and 3Dimensional architecture Weak Forces q van der Waals: 0.4-4.0 kJ/mole q Hydrogen bonds: 12-30 kJ/mole q Ionic bonds: 20 kJ/mole q Hydrophobic interactions: < 40 kJ/mole Ionic bonds in biological molecules Protein strand Two Important Points About Weak Forces Structural complementary Biomolecular Recognition is Mediated by Weak Chemical Forces Weak Forces Restrict Organisms to a Narrow Range of Environmental Conditions Biochemistry 2/e - Garrett & Grisham Denaturation and renaturation of the intricate Structure of a protein Copyright © 1999 by Harcourt Brace & Company Metabolism is the organized release or capture of small amounts Of energy in processes whose overall change in energy is large Biochemistry as a Biological Science Enzyme catalyze metabolic reactions A Biomolecular Hierarchy Simple Molecules are the Units for Building Complex Structures - Metabolites and Macromolecules - Organelles - Membranes “The Unit of Life is the Cell” ! Carbonic anhydrase Organization and Structure of Cells Prokaryotic cells A single (plasma) membrane no nucleus or organelles Eukaryotic cells much larger in size than prokaryotes 103-104 times larger! Nucleus plus many organelles ER, Golgi, mitochondria, etc. Page 7 Fig 1-4 Phylogenetic tree. Figure 1-1 Scale drawings of some prokaryotic cells. Page 4 Page 4 Figure 1-2 Schematic drawing of a prokaryotic cell. 1.5 What is the Organization and Structure of Cells? Fig. 1-5 Schematic diagram of an animal cell accompanied by electron micrographs of its organelles. Page 7 Page 11 Fig. 1-9 Drawing of a plant cell accompanied by electron micrographs of its organelles 1.5 What is the Organization and Structure of Cells? Figure 1.22 Electron micrograph of a chloroplast. Figure 1.22 Electron micrograph of a Golgi body. 1.5 What is the Organization and Structure of Cells? Figure 1.22 Electron micrograph of a nucleus 1.5 What is the Organization and Structure of Cells? 1.5 What is the Organization and Structure of Cells? 1.5 What is the Organization and Structure of Cells? 1.6 What Are Viruses? 1.6 What are Viruses? Figure 1.23 Viruses are genetic elements enclosed in a protein coat. Viruses are not free-living organisms and can reproduce only within cells. Viruses show an almost absolute specificity for their particular host cells, infecting and multiplying only within those cells. Viruses are known for virtually every kind of cell. Shown here are examples of (a) an animal virus, adenovirus; (b) bacteriophage T4 on E.coli; and (c) a plant virus, tobacco mosaic virus. Figure 1.24 The virus life cycle. Viruses are mobile bits of genetic information encapsulated in a protein coat. Function of organelles of eukaryotes v v v v v v v Mitochondria Endoplasmic reticulum Golgi complex Nucleus Lysosomes Chloroplasts peroxisomes Figure 1-12Embryonic development of a fish, an amphibian (salamander), a bird (chick), and a mammal (human). Page 14 Page 13 Fig. 1-11 Evolutionary tree indicating the lines of descent of cellular life on Earth.