PhaseChangeExperimentwithIce-1
advertisement

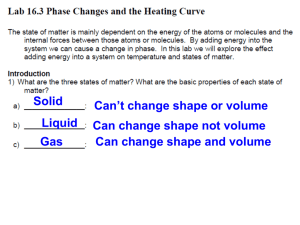
Name:______________ Monitoring Phase Change of Water Exploration Introduction/Background: Kinetic and Potential Energy in Phase Changes Graphs are a way of communicating energy changes. We start with a solid phase, using ___________ as a solid. When water freezes, it forms a ____ - dimensional structure that prevents the water molecules from getting too ___________ to one another. Each oxygen atom is associated with _____ hydrogen atoms, two by ___________ bonds, and two by _________________________ forces. Intermolecular forces are unusually ____________ in water due to the Hydrogen bonding. This is evident in water’s relatively high _________________ point and boiling point. A steady addition of heat to frozen water molecules causes them to ___________ more, which raises the average kinetic energy, and this raises the __________________ . At the melting point, the intermolecular forces are no longer strong enough to withstand the vibrations. The thermal energy is now used to overcome the forces that lock the ice molecules together, and as a result, _________________ energy increases. This causes a _______________ change, solid to liquid. The temperature does not _________. This energy is called the molar _________ of _____________. (Hfus) Once all the solid has melted, the further addition of heat causes the average _____________ energy to increase. We measure this increase in kinetic energy as an increase in _____________________. At the boiling point, the temperature does not rise, so we know that the average kinetic energy remains constant. Now, the addition of heat works against the ___________________ forces which hold the molecules together in the liquid. Molecules then _______________ , storing the added energy as _____________________ energy. Free of the intermolecular forces, the molecules fly apart as a ________ . Once again, the added heat raises the potential energy of the molecules, and a _________________ change has occurred, liquid to gas. The energy absorbed is called the molar _________ of _______________. (Hvap) Purpose/Goal: We will monitor the potential and kinetic energy absorbed by the ice as it changes to water, and then to boiling water in order to graph our results and calculate the heat absorbed. Materials: Procedure: Measure the mass of your empty beaker and record. Collect a sample of ice from your teacher and re-measure the mass. Record. (Subtract to find the mass of the ice!) Read the temperature (°C) of the ice and record as the initial temperature. Simultaneously set the beaker on the hot plate (medium heat!) and start the stop watch. Read and record the temperature EVERY minute. Stir continuously to keep a more accurate temperature reading. You may stop after the water has been vigorously boiling for at least four minutes. Graph your results as a temperature-time graph. Calculate the TOTAL energy absorbed (calories) by the mass of your ice. DATA/Results: Mass of empty Beaker: _________g Time (min) Temperature (°C) Mass of Beaker + Ice: __________g Time (min) Temperature (°C) Mass of Ice: _________g Time (min) 0 11 22 1 12 23 2 13 24 3 14 25 4 15 26 5 16 27 6 17 28 7 18 29 8 19 30 9 20 31 10 21 32 Temperature (°C) Calculations: 1. Knowing that you had ___________ grams of ice, how much energy did it take to overcome the Heat of Fusion? 2. How much energy did it take to raise the temperature of the water from 0 °C to ________ °C? Max T 3. How much energy would it take to overcome the Heat of Vaporization? Did your sample completely vaporize within the time tested? GRAPH: Prepare a Temperature-Time graph that demonstrates your group’s data. Plot each minute’s temperature, and then draw a “best fit” line to better see your phase change curve. Name:______________ Monitoring Phase Change of Water Exploration Introduction/Background: (Teacher Key) Kinetic and Potential Energy in Phase Changes Graphs are a way of communicating energy changes. We start with a solid phase, using ____ice_______ as a solid. When water freezes, it forms a __3__ - dimensional structure that prevents the water molecules from getting too ____close_______ to one another. Each oxygen atom is associated with __4___ hydrogen atoms, two by __covalent_____ bonds, and two by _________intermolecular______ forces. Intermolecular forces are unusually ______strong______ in water due to the Hydrogen bonding. This is evident in water’s relatively high __melting___ point and boiling point. A steady addition of heat to frozen water molecules causes them to _____vibrate______ more, which raises the average kinetic energy, and this raises the ________temperature__________ . At the melting point, the intermolecular forces are no longer strong enough to withstand the vibrations. The thermal energy is now used to overcome the forces that lock the ice molecules together, and as a result, _______potential____ energy increases. This causes a ______phase______ change, solid to liquid. The temperature does not ___change______. This energy is called the molar _____Heat____ of _____Fusion___. (Hfus) Once all the solid has melted, the further addition of heat causes the average _____kinetic______ energy to increase. We measure this increase in kinetic energy as an increase in ________temperature_________. At the boiling point, the temperature does not rise, so we know that the average kinetic energy remains constant. Now, the addition of heat works against the _______intermolecular________ forces which hold the molecules together in the liquid. Molecules then ______detach______ , storing the added energy as ______potential_______ energy. Free of the intermolecular forces, the molecules fly apart as a ____gas____ . Once again, the added heat raises the potential energy of the molecules, and a _____phase_______ change has occurred, liquid to gas. The energy absorbed is called the molar _Heat_ of ______Vaporization_____. (Hvap) Purpose/Goal: We will monitor the potential and kinetic energy absorbed by the ice as it changes to water, and then to boiling water in order to graph our results and calculate the heat absorbed. Materials: Procedure: Measure the mass of your empty beaker and record. Collect a sample of ice from your teacher and re-measure the mass. Record. (Subtract to find the mass of the ice!) Read the temperature (°C) of the ice and record as the initial temperature. Simultaneously set the beaker on the hot plate (medium heat!) and start the stop watch. Read and record the temperature EVERY minute. Stir continuously to keep a more accurate temperature reading. You may stop after the water has been vigorously boiling for at least four minutes. Graph your results as a temperature-time graph. Calculate the TOTAL energy absorbed (calories) by the mass of your ice.