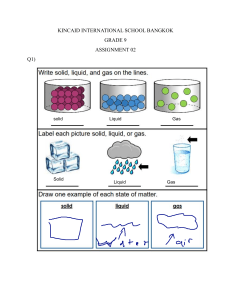
1) It is important to understand the difference between _______________ and ___________________ changes. 2)_______________ changes are usually about states and _________________ changes happen on a molecular level. 3) When you have two or more molecules that interact do you get a physical change, or do you get a chemical change? _______________ 4) when you step on a can and ______________ it you have forced a __________________ change. 5) Explain to me why we only created a physical change to the can when we crushed it. 6) After you crush a can the _________________ in the can are still the same __________________. No _________________ bonds were created or broken. 7) Can you find the H2O on the periodic table? __________ 8) Is melting Ice a physical change or a chemical change? ____________________ 9) Is freezing water in to Ice a physical change or a chemical change? ____________________ 10) A change in __________________ and _____________ can cause a ________________ change in the _____________ molecules. 11) Draw a picture of a physical change. 1) Do chemical changes happen on a large scale or small scale? _______________________ 2) While some ________________ show obvious ______________ changes, such as a __________ change, most _____________ changes are not ________________. 3) The chemical change between as hydrogen peroxide becomes water can not be seen why? _______ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ 4) Because it is hard to see a reaction of H2O2 becoming water you might still see ______________ as evidence of the chemical change. 5) Melting a _____________________ cube is a _______________________ change because the _______________________ is still _______________________. 6) If we burn the sugar is it a chemical change or a physical change? _____________________ 7) __________________ will eventually _______________ when it is exposed to _____________________ gas in the _______________. 8) You can watch the process of _________________ happen over a _______________ period of time. 9) What is the symbol for iron on the periodic table? __________ 10) Draw a picture of the oxidation process.