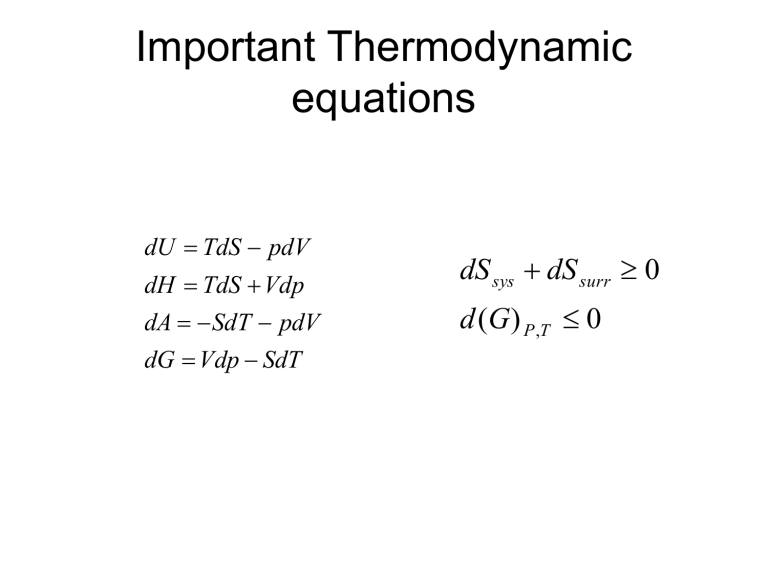
Important Thermodynamic equations dU TdS pdV dH TdS Vdp dS sys dS surr 0 dA SdT pdV d (G ) P ,T 0 dG Vdp SdT Conditions for equilibrium between Phases (1) dS U ,V 0 For isolated system, dS dS dS 0 dq dq 0 T T T T (Thermal Equil.) If the temperatu re is not same, heat will flow from one phase to another. (2) dAV ,T 0 At constant v olume and temperatu re, p dV p dV 0 p p (Mechanica l Equil.) At constant V and T, the pressure of the two phases should be the same. If not, one phase would increase in volume other. at the expense of (3) dG T , P 0 For an open system, G f T , P, n dG SdT Vdp i dni At constant t emperature and Pressure, dG T , P i dni 0 i dni i dni 0 i i (Chemical Equil.) At constant Temp. and Pressure, the chemical potential of a component should be the same in two phases. If not, the transfer of that component will take place from one phase to other. Various Equilibrium conditions Capacity Factor S Intensity Factor T Equilibrium Condition T T At const. U,V V P p p At const. V,T ni i i i At const. P,T Chemical Potential dG SdT Vdp i dni G n T , P ,n j i dU TdS pdV i dni U H A i n S ,V ,n j n S , p ,n j n T ,V ,n j Intergration of function dU TdS PdV i dni Here, U, S, V and n all are extensive function, U TS PV i ni G H TS U PV TS G i ni Phase changes of pure component solid G liquid vapour N i 1 i ni For one component system, G n G molar Gibb' s Function n For a spontaneou s reaction, dG T , P 0 i dni i dni 0 i i 0 i i A species diffuses spontaneou sly from the phase where its chemical potential is higher to a phase where its chemical potential is lower. Physical meaning of Chemical potential • Tendency of a system to give particles. Temperature dependence of phase stability dG SdT Vdp G S T p Gm Sm T p Sm T p (negative S m (g) S m (l) S m (s) quantity) Ice-skating • As we glide across the ice, we exert pressure on the thin blade, and are therefore creating a small stream of water in our path by melting that ice. The water between the blade and the ice is what we really glide across. Response of melting temperature to the applied pressure Vm p T Relationship between equilibrium temperature and pressure e.g. Solid-liquid equilibria Solid Liquid Suppose at Pressure P , the melting temperatu re is T 1 1 So at T and P , the chemical potential of component is same in both phases 1 1 s (i) l Now on changing the Pressure to P , the melting temperatu re changes to T 2 2 s' ' (ii) l (ii) -(i) s' s ' l l ds d l S dT V dP S dT V dP L L S S (S S )dT (V V )dP L S L S dP S dT V Relation between dp and dT a ( p , T ) a ( p , T ) At b, the chemical potentials changes but are still equal. b ( p , T ) b ( p , T ) d d S , m dT V , m dp S , m dT V , m dp V ,m V , m dT trs S dp dT trsV S ,m S , m dp (Clapeyron equation) The solid –liquid boundary ve dp fus H dT T fusV ve but small ve, large Steep curve Liquid –Vapour boundary dp vap H ve dT T vapV ve, large ve, small vapV Vm (g) dp dT vap H RT T p vap H d ln p dT RT 2 (Clausius Clapeyron Equation) Solid-Vapour Boundary dp sub H vap H ve dT T subV T vapV ve, large ve Steeper th an liquid - vap 1 bar Elevation of boiling point • Boiling point of water is raised if the pressure above water is increased; it is lowered if the pressure is reduced. This explains why water boils at 70 °C up in the Himalaya Elevation of boiling point • An application of this effect is the pressure cooker. A pressure cooker traps steam inside it, raising the pressure to about twice the atmospheric pressure. As a result, the water boils at 120 °C. Food cooks quicker at the higher temperature. There is a safety valve on the cover which opens if the pressure is too high. d ln p vap H dT RT 2 p 2 vap H ln p1 R 1 1 T1 T2 (Clausius Clapeyron Equation) Autoclave • Sterilization of both materials and contaminated media is an essential process in a laboratory for in vitro cultures. • This sterilization is usually done in a device called an autoclave. • Essentially, an autoclave is a container that exposes the material to be sterilized to temperatures above the boiling point of water, which is achieved by increasing pressure. H fus 6010 Q. It has been suggested that the surface melting of ice plays a role in enabling speed skaters to achieve peak performance. Carry out the following calculation to test this hypothesis. At 1 atm pressure, ice melts at 273.15 K, ΔHf =6010 J mol-1, the density of ice is 920 Kg m-3, and the density of liquid water is 997 kg m-3. (a) What pressure is required to lower the melting temperature by 5.0 ºC ? (b) Assume the width of the skate in contact with ice has been reduced by sharpening to 25*10-3 cm, and that the length of the contact area is 15 cm. If the skater of mass 85 kg is balanced on one skate, what pressure is exerted at the interface of the skate and the ice ? ©What is the melting point of ice under this pressure? (d) If the temperature of ice is -5 ºC, do you expect melting of ice at ice-skate interface to occur ? H of vaporization of water is 539.4 cal/g at the normal boiling point. Many bacteria can survive at 100 C by forming spores. Hence autoclaves used to sterlize medical and laboratory instruments are pressurized to raise the boiling point of water to 120 C. What will be the pressure required to increase the boiling point of water to 120 ºC ? Q. Ar has normal melting and boiling points of 83.8 and 87.3 K; its triple point is at 83.8 K and 0.7 atm, and its critical temperature and pressure are 151 K and 48 atm. State whether Ar is a solid, liquid, or gas under each of the following conditions (a) 0.9 atm and 90 K (b) 0.7 atm and 80 K (c) 0.8 atm and 88 K (d) 0.8 atm and 84 K (e) 1.2 atm and 83.5 K. Q. Dry ice is frozen carbondioxide. A block of dry ice has a surface temperature of -78.5 C. If you want to send something frozen across the country, you can pack it in dry ice. It will be frozen when it reaches its destination, and there will be no messy liquid left over like you would have with normal ice. Explain this phenomenon with the help of a phase diagram? The vapor pressure of zinc varies with temperature as log p (mmHg)= -6850/T – 0.755log T + 11.24 and that of liquid Zn as log p (mmHg) = -6620/T -1.255 log T + 12.34 calculate (a) boiling pt of Zn. (b) the triple point. © the heat of evaporation at the boiling point. (d) heat of fusion. (e) the difference in Cps of solid and liquid Zn. The effect of applied pressure on vapor pressure At equilibriu m, (l ) ( g ) For any change that preserves equili. d (l) d Vm (l) dP (v) RT dp p Vm (l) P RT ln p2 p1 When pressure is applied to a condensed phase, its vapour pressure rises. Phase Rule F=C-P+2 C = number of component Degree of Freedom • Number of independent intensive variables needed to specify its intensive state. T, P and mole fraction in each phase. Total number of intensive variable = pc +2 Mole fraction of components in each phase must be one Number of relation =number of phases= p For chemical equilibrium, Chemical potential of individual component will be same in each of the phases. 1 1 1 1 ....... In each of the component (p-1) relation for each of the component Total number of relation for chemical equilibrium = c(p-1) Degree of Freedom = pc + 2 – p - c(p-1) =c–p+2 What happens to boiling point or freezing point of a liquid in the presence of a solute? What happens to chemical potential of a liquid in the presence of solute? For pure solvent A (l) A (g) RT ln p A A A In presence of a solute A (l) A (g) RT ln p A A A RT ln A A p p A RT ln x A A (Raoult' s law Chemical potential of a liquid on addition of non - volatile p p* A xA ) decreases solute. There is elevation of boiling point upon addition of a solute. There is depression of freezing point upon addition of a solute.




