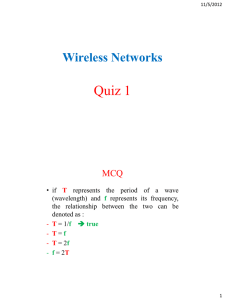
Chemistry Notes To get a clear picture of how scientists solved the periodic table puzzle, you will complete an activity using only the data available to scientists in the 1860s. Putting that data in order by atomic weight (as Newlands did), you'll look for groups of elements that seem to have similar properties. After you identify those groups, you'll try to spot patterns of similarity in their order. The modern periodic table arranges elements by atomic number into 7 rows and 18 columns. Each element is identified by a lettered symbol placed in a box, or tag, with information about the element. The atomic number typically appears at the top of the box. Among the main group elements, the elements in group 1A at the left of the periodic table, excluding hydrogen, are known as the alkali metals. These highly reactive elements tend to form oxygen–containing compounds that dissolve in water to produce solutions that are strongly alkaline, or basic, on the pH scale. The group 2A elements are also metals that form alkaline–oxygen compounds. These elements don't dissolve in water as readily as the group 1A elements, however. As a result, they're often found in earthy soil deposits and are called the alkaline earth metals. Let's go over some of the basic concepts related to waves. Look at the two waveforms shown here. Both of them start at the rest line from point O, reach the highest point P, pass through the rest line again, reach the lowest point, and then come back to rest again. This makes one complete cycle, which a wave repeats over and over. The highest point of a wave is called the crest. The points P and R represent the two crests in consecutive wave cycles. The height of P from the rest line of a wave is called its amplitude. Notice the different amplitudes of the two waves. The distance between two consecutive crests, such as P and R, is called the wavelength of the wave. Note that the wavelength of the first wave (about 2.5 units) is longer than that of the second wave (about 1.5 units). A wavelength is represented by the Greek letter lambda (λ). The number of wave cycles that pass through a specific point within a given time period is the frequency of the wave. It is represented by the Greek letter nu (n) and the unit of measurement is Hertz (Hz)—representing cycles per second. Since it has a shorter wavelength, the second wave has a higher frequency than the first wave.