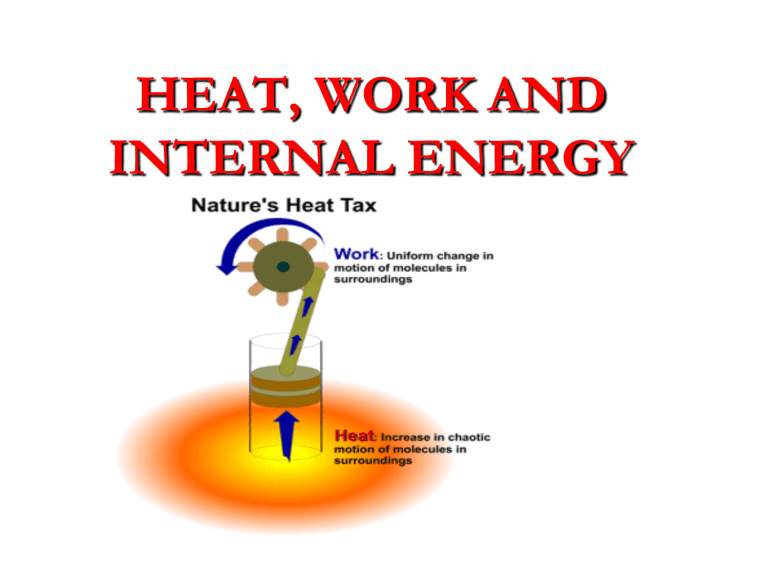
HEAT, WORK AND INTERNAL ENERGY Objectives What is the ideal gas GAS-PROPERTIES • https://phet.colorado.edu/en/simulation/lega cy/balloons-and-buoyancy https://www.youtube.com/watch?v=BTyQj39ZbMs Work done on a gas Won gas =-pV • • • • IN SI system of units, P : Pascal (Pa), V : m3, W : Joule (J) Problem 1 The volume of an ideal gas changes from 0.40 to 0.55 m3 although its pressure remains constant at 50 000 Pa. What work is done on system(gas) by its environment? a. 7 500 J b. 200 000 J c. 7 500 J d. 200 000 J Problem 2 The volume of an ideal gas changes from 0.40 to 0.55 m3 although its pressure remains constant at 50 000 Pa. What work is done by the gas on its environment? a. 7 500 J b. 200 000 J c. 7 500 J d. 200 000 J PROBLEM2 • In an isobaric process 4.5 104 J of work is done on a quantity of gas while its volume changes from 2.6 m3 to 1.1 m3. What is the pressure during this process? a. 1.2 104 Pa b. 2.4 104 Pa c. 3.0 104 Pa d. 4.1 104 Pa problem3 • Calculate the work done by a gas that expands from 0.020 m3 to 0.80 m3 at constant atmospheric pressure. • How much work is done by the environment on the gas when it expands this much? Work Done by a gas from PV-graph • Area under pressure-volume curve is the work done • W = P∆V • W = Area under PV graph. The figure given below represents p-V diagram of different stages of a thermodynamic process. Calculate the work done in each stage and also the net work done in the complete cyclic process. Calculate the work done on the gas from D to E to F 1-from D to E 2-from E to F 3-from F to D 4-total work Example 12.2 Find the numeric value of the work done on the gas in (a) Figure 12.4a and (b) Figure 12.4b. Solution to Example 12.2 Quick Quiz By visual inspection, order the PV diagrams shown in Figures below from the most negative work done on the system to the most positive work done on the system. (a)a, b, c, d (b) a, c, b, d (c) d, b, c, a (d) d, a, c, b Quick Quiz Objectives Gas processes Gas process: describes how a gas gets from one state to another Isothermal: process occurs at constant temperature Isobaric: process occurs at constant pressure isovolumetric/Isometric/Isochoric: process occurs at constant volume Adiabatic: process is insulated, no heat energy enters or leaves system (Q = 0) Isobaric Process (constant pressure) P T1 T2 T 3 Isobaric Expansion Isobaric Contraction P = 0 (constant P) V Isothermal Process (constant temperature) P T1 T2 T 3 Initial State of Gas Isothermal Process Final State of Gas Gas “isotherms” T = 0 (constant T) V Isometric Process (constant volume) P T1T2 T 3 V = 0 (constant V) V Adiabatic process (insulated) P Temperature, pressure, and volume all change in an adiabatic process. T isotherm slope of an adiabatic curve steeper than an isothermal curve. adiabat Q = 0 (no heat enters or leaves) V Why the slope of an adiabatic curve steeper than an isothermal curve.? • For an adiabatic compression, the pressure change is due to - reduction in volume - increase in temperature (since there's no heat lost from the system, the temperature rises when you do work on the system); this means an increase in average particle speed. • For an isothermal compression, the pressure change is due to - reduction in volume alone. The temperature does not increase, heat energy is removed from the system (to keep the temperature constant). So the particles aren't moving as fast, compared to the adiabatic change. The change in pressure (for the same volume change) is therefore higher for an adiabatic changes, making the graph steeper. Problem 2 • During an isobaric process which one of the following does not change? a. volume b. temperature c. internal energy d. pressure Problem 2 • During an isothermal process which one of the following does not change? a. volume b. temperature c. internal energy d. pressure Problem 4 • On a P-V diagram, an ____ process is represented by a horizontal line. a. isobaric b. isothermal c. isovolumetric d. adiabatic Problem 4 • On a P-V diagram, an ____ process is represented by a vertical line. a. isobaric b. isothermal c. isovolumetric d. adiabatic Problem 3 • Area on a P-V diagram has units associated with: a. energy. b. momentum. c. temperature. d. change in temperature. The first law of Thermodynamic • https://www.youtube.com/watch?v=qVAvmie RM1E The First Law of Thermodynamics states that : The internal energy of a system changes from an initial value Ui to a final value Uf due to heat added (Q) and work done by the system (W) U = Uf – Ui = Q – W • Q is positive when the system gains heat, and negative when the system loses heat. • W is positive when it is done BY the system, and negative when it is done ON the system 15-1 The First Law of Thermodynamics An amount of heat equal to 2500 J is added to a system, and 1800 J of work is done on the system. What is the change in internal energy of the system? Q = +2500 J W = -1800 J Δ U = Q – W = +2500 – (-1800) = 4300 J 15-1 The First Law of Thermodynamics What would be the internal energy change if 2500 J of heat is added to the system and 1800 J of work is done by the system (i.e. as output)? Q = +2500 J W = +1800 J Δ U = Q – W = +2500 – (+1800) = 700 J First law and thermodynamic processes https://edpuzzle.com/media/59d282df9750b4 4afb418d45 problem1 • A system is acted on by its surroundings in such a way that it receives 50 J of heat while simultaneously doing 20 J of work. What is its net change in internal energy. a. 70 J b. 30 J c. zero d. 30 J Problem2 • According to the first law of thermodynamics, the sum of the heat gained by a system and the work done on that same system is equivalent to which of the following? a. entropy change b. internal energy change c. temperature change d. specific heat Problem2 • In an isothermal process for an ideal gas system (where the internal energy doesn't change), which of the following choices best corresponds to the value of the work done on the system? a. its heat intake b. twice its heat intake c. the negative of its heat intake d. twice the negative of its heat intake Problem4 In an isovolumetric process by an ideal gas, the system's heat gain is equivalent to a change in: a. temperature. b. volume. c. pressure. d. internal energy. Internal combustion engine Sample problem Problem1 A heat engine receives 6 000 J of heat from its combustion process and loses 4 000 J through the exhaust and friction. What is its efficiency? ( ANSWER:A) a. 33% b. 40% c. 67% d. 73% Problem2 • If a heat engine has an efficiency of 30% and its input energy is 6000J , what is useful work done by it ? a. 1 800 J b. 2 400 J c. 2 000 J d. 3 000 J Problem3 • A heat engine exhausts 3 000 J of heat while performing 1 500 J of useful work. What is the efficiency of the engine? (ANSWER :B) a. 15% b. 33% c. 50% d. 60%








