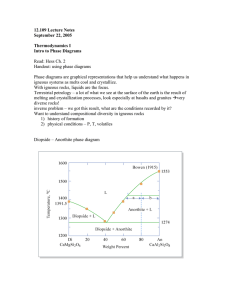
European Microelectronics Packaging Conference 10-13 September 2017, Warsaw, Poland www.empc2017.pl Liquid Solid Diffusion (LSD) Bonding A novel joining technology Andreas Larsson TECHNI AS, Applied physics dep. USN – University College of Southeast Norway, Dep. of Microsystems Borre, Norway andreas.larsson@techni.no ala@usn.no Torleif A. Tollefsen TEGma AS Oslo, Norway Knut E. Aasmundtveit USN – University College of Southeast Norway, Dep. of Microsystems Borre, Norway Ole Martin Løvvik SINTEF Materials and Chemistry Oslo, Norway II. CONCEPT Abstract—Liquid Solid Diffusion (LSD) bonding is a novel joining technology forming solid and liquid two-phase field joints from simple binary systems. LSD creates joints that are applicable at temperatures above the melting temperature of the material composition of the initial bond. Liquid and solid interdiffusion transform the joint composition and microstructure. The final joint is characterized by incongruent re-melting forming a coherent continuous porous solid solution phase with liquefied pores. This article briefly describes the methodology and presents experimental results based on the Au–Ge system. High quality joints were created. The effective melting point was increased by more than 200 °C above the initial eutectic melting point of the system. Keywords—Bonding, high temperature, Au–Ge I. INTRODUCTION Joining of materials or components is an ancient art emanating many hundreds of years back in time [1], [2]. In more recent time, joining metals have become an art of greatest importance. Joining of metals have enabled man to create extraordinary things and devices such as the space shuttle and enabling use of the integrated circuit. Some of the most advanced forms of joining technologies in use today is within assembly of complex electronic systems (often referred to as bonding). Such technologies include; soldering, brazing, welding, contact melting [3], [4], thermo-compression, ultrasonic, solid liquid inter-diffusion (SLID) [5]–[8] / transient liquid phase (TLP) [9] and sintering [10]. This paper presents a brief description of a novel joining technology called Liquid Solid Diffusion (LSD) bonding. Like other comparable joining technologies, such as transient liquid phase (TLP) bonding [9], LSD creates joints that are applicable at temperatures above the melting temperature of the material composition of the initial bond. Unlike TLP and other similar joints, LSD joints comprise a liquid phase at high temperatures. This report presents initial experimental results demonstrating an increase in effective melting temperature of the joint after bonding. It is well known that binary eutectic material systems are partially solid and liquid when in the two-phase field region between solidus and liquidus in the phase diagram [11]. During solidification of off-eutectic compounds, a mushy mixture is formed in the aforementioned two-phase field region, when the temperature is between the liquidus temperature and the solidus temperature. Much like in a slushy were solid ice particles float in a liquid. Therefore, it is often referred to as the mushy region. When reheated after complete solidification, the same mushy structure normally reforms. This makes the compound easily reworkable, which is used in some applications such as soldering pipes with plumbers’ solder. It was envisaged that it was possible to switch place of the liquid and solid phases forming a continuous and coherent porous structure with liquefied pores at elevated temperatures, instead of a mushy state. This could be done by diluting the compound, preferably close to the maximum solubility limit, and annealing it such that phase segregation change the microstructure and morphology of the joint. When reheated the segregated solute then reacts locally with the solvent. At the eutectic temperature, a liquid is formed at the interface between the two phases. The reaction continues until all the solute has been consumed, forming a eutectic liquid domain. The surrounding material then comprise a solid single phase structure. The solid phase may be used to maintain structural integrity between to adjoined components at a temperature significantly above the eutectic melting point of the material system. III. METHODOLOGY A. Materials and fabrication Dummy samples were fabricated within the binary Au–Ge system. Square chips and substrates, 3.6 mm2 and 15.2 mm2, were diced of 525 µm thick silicon wafers with a 150 nm thick TiW, and a 2.8 µm thick Au layer on the bond surface. A 25 µm thick eutectic Au–Ge preform was then sandwiched between chips and substrates, cf. Fig. 1. © IMAPS / EMPC 2017 1 European Microelectronics Packaging Conference The chip and preform were manually aligned on top of the substrate forming a symmetrical Si / Au / Au–Ge / Au / Si structure and placed onto a heater. The stack was clamped together and the bond process was carried out in vacuum. The temperature was raised above the eutectic melting temperature (361 °C) to around 380 °C and then lowered to around 330 °C and maintained at this temperature for up to 4 hours. The applied pressure was approximately 2 MPa. The pressure ensured squeeze-out of excess material from the bond interface. This minimized the active volume of eutectic material for the interdiffusion process to form the final joint. The pressure also secured a thermomechanical contact between chip and substrate. Reference samples forming a regular eutectic joint were also fabricated. The annealing step at 330 °C, transforming the microstructure, was omitted for the reference samples. 10-13 September 2017, Warsaw, Poland www.empc2017.pl Fig. 1 Illustration of the investigated sample components. B. Characterization An integrity test of the fabricated joints was performed by clamping the fabricated samples on a heater (Watlow; Ultramic 600) in vacuum. A weight was applied to the structure creating a shear force over the bond in the range of 20-50 kPa. The temperature was then raised at a rate of 30 °C/min up to a maximum of 600 °C or until the chip detached from the substrate. Cross-sections were analyzed with use of optical microscopy (II Neophot 32) and scanning electron microscopy (SEM) (Hitachi SU8320). Samples were prepared for cross-section by dicing and Ar ion-milling (Hitachi IM4000). The joint composition was evaluated by energydispersive X-ray spectroscopy (EDX) (Oxford Silicon Drift Detector- XmaxN). IV. RESULTS & DISCUSSION Fig. 2 Ongoing detachment experiment. Hot plate glowing red hot at ca. 570 °C. A. Detachment temperature The fabricated bonds demonstrated structural integrity up to the maximum temperature of the hot plate of 600 °C, cf. Fig. 2. This demonstrates that there exists a solid, continuous and coherent structure adjoining the two components structurally at temperatures above the eutectic temperature of 361 °C. The reference samples detached around 330 ± 10 °C. Comparable regular eutectic Au–Ge joints have been reported to have a significant shear strength of tens of MPa at temperatures around 300 °C and above [12]–[14]. This discrepancy is still unclear. B. Joint quality and composition Cross-sections of the joint showed high quality joints with limited voiding. Analysis by SEM and EDX of the joint showed that it mainly comprised an overall Au rich Au–Ge mixture with an overall Ge concentration of around 8 at%, cf. Fig. 3. It was further observed that the desired morphology had been achieved, cf. Fig. 4. I.e. the β-phase (Ge) had segregated and was isolated into small domains inside an otherwise homogenous α-phase (Au with dissolved Ge) without any significant regions showing a eutectic morphology. Fig. 3 The phase diagram of the Au–Ge material system [15]. The dashed blue line indicates the overall composition of the fabricated LSD joints. © IMAPS / EMPC 2017 2 European Microelectronics Packaging Conference 10-13 September 2017, Warsaw, Poland www.empc2017.pl bonding : Cu – Sn , Au – Sn and Au – In,” in Electronics System-Integration Technology Conference (ESTC), 2014, no. Eq 1, pp. 1–6. [8] A. Larsson, T. A. Tollefsen, O. M. Løvvik, and K. E. Aasmundtveit, “Ni – Sn solid liquid interdiffusion ( SLID ) bonding – Process , bond characteristics and strength,” in 6th Electronic System-Integration Technology Conference (ESTC), 2016. Fig. 4 SEM micrograph of a cross-section of a fabricated liquid solid diffusion (LSD) joint. Light grey regions represent the α phase (Au), and the small and isolated dark grey regions the β phase (Ge). V. CONCLUSIONS A brief description of a novel joining method called liquid solid diffusion (LSD) bonding was presented. Detachment experiments demonstrated joints with structural integrity at temperatures significantly higher than the initial melting temperature of the system. This indicates a solid, continuous and coherent structure adjoining the two components structurally at high temperatures. Cross-sectional analysis showed the joint to be a mixture with about 8 at-% Ge (βphase). The β-phase had segregated into isolated small local domains in a homogenous matrix of α-phase (Au). VI. ACKNOWLEDGEMENT The authors would like to acknowledge the Norwegian Research Council for supporting this project (Project No.: 244915). VII. REFERENCES [1] C. S. Smith, A history of metallography - The development of ideas on the structure of metals before 1890, 1st ed. Chicago: The University of Chicago Press., 1960. [2] C. S. Smith, A search for structure. MIT Pr, 1981. [3] M. Lacroix, “Contact melting of a phase change material inside a heated parallelepedic capsule,” Energy Convers. Manag., vol. 42, no. 1, pp. 35–47, 2001. [4] [9] G. O. Cook and C. D. Sorensen, “Overview of transient liquid phase and partial transient liquid phase bonding,” J. Mater. Sci., vol. 46, no. 16, pp. 5305–5323, 2011. [10] V. R. V. R. Manikam and K. Y. K. Y. K. Y. Cheong, “Die Attach Materials for High Temperature Applications: A Review,” Components, Packag. Manuf. Technol. IEEE Trans., vol. 1, no. 4, pp. 457–478, 2011. [11] H. Okamoto, Desk Handbook: Phase Diagrams for Binary Alloys, 2nd ed. United States of America: ASM International, 2010. [12] A. Drevin-Bazin, F. Lacroix, and J. F. Barbot, “SiC die attach for high-temperature applications,” J. Electron. Mater., vol. 43, no. 3, pp. 695–701, 2014. [13] S. Tanimoto, K. Matsui, Y. Murakami, H. Yamaguchi, and H. Okumura, “Assessment of Au-Ge Die Attachment for an Extended Junction Temperature Range in Power Applications,” in International Conference and Exhibition on High Temperature Electronics, 2010, no. HiTEC, pp. 32–39. [14] W. Sabbah, S. Azzopardi, C. Buttay, R. Meuret, and E. Woirgard, “Study of die attach technologies for high temperature power electronics: Silver sintering and gold-germanium alloy,” Microelectron. Reliab., vol. 53, no. 9–11, pp. 1617–1621, 2013. [15] H. Okamoto and T. B. Massalski, “The Au-Ge (GoldGermanium) System,” Bull. Alloy Phase Diagrams, vol. 5, no. 6, pp. 601–610, 1984. a. P. Kryshtal, R. V. Sukhov, and a. a. Minenkov, “Critical thickness of contact melting in the Au/Ge layered film system,” J. Alloys Compd., vol. 512, no. 1, pp. 311–315, 2012. [5] L. Bernstein, “Semiconductor Joining by the SolidLiquid-Interdiffusion (SLID) Process,” J. Electrochem. Soc., vol. 113, no. 12, pp. 1282–1289, 1966. [6] T. A. Tollefsen, A. Larsson, M. M. V. Taklo, A. Neels, X. Maeder, K. Høydalsvik, D. W. Breiby, and K. Aasmundtveit, “Au-Sn SLID Bonding: A Reliable HT Interconnect and Die Attach Technology,” Metall. Mater. Trans. B, vol. 44, no. 2, pp. 406–413, 2013. [7] K. E. Aasmundtveit, T. Luu, A. B. Vardøy, T. A. Tollefsen, K. Wang, and N. Hoivik, “High-Temperature Shear Strength of Solid-Liquid Interdiffusion ( SLID ) © IMAPS / EMPC 2017 3