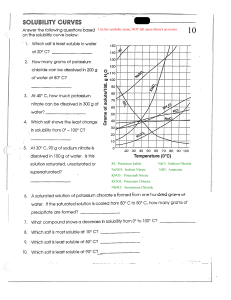
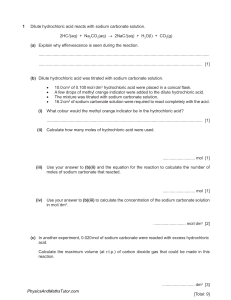
Covid-19 Online Semester Exam Federal Urdu University of Arts, Science and Technology Gulshan-e-Iqbal Campus, Karachi BS 2nd Year Semester IV 2021 (Regular + Ex) Analytical Chemistry (CHEM-411) Name _______________ Roll Number ______________ Class ____________ Major Department _________________ Regular or Ex Student ____________ Instructions: - (i) Attempt all questions in sequence. Total Marks: 70 (Regular +Ex) (ii) All questions carry equal marks (3.5 each). Time: 9:00 am to 3:00 pm Note: PDF Version of Answer Sheet File must Email before 3:00 pm. Late Email will not consider for checking. 1. (a) What are the major types of analytical balance? Also mention their capacity and precision. (b) Why does bouncy error occur and when it will be significant? (c) Explain pipets and its major types along diagrams. (d) A bottle weighed 5.6700 g empty and 7.9300 g after introduction of a solvent with a density of 0.89 g/cm3. The balance was equipped with stainless steel masses (d=8.0 g/cm3). Correct the mass of the sample for the effects of buoyancy. 2. (a) Write the 3 differences between end point and equivalent point in volumetric titration. (b) How many grams Na2WO4 (sodium tungstate) should be weighed out to prepare 500 mL of a 0.200 M solution? (c) Justify the following statements. i- How would you identify, glassware is cleaned? ii-Why we really deal with masses rather than weights? iii-Transfer pipets are more accurate than measuring pipet. iv-Equivalence Point is of more concerned as compare to end point. (d) Calculate the number of moles in 250 mg Na2SO4. 3. (a) Identify primary standard and secondary standard chemicals separately. HNO3, Oxalic acid, KCl, pure metals (Zn, Cu, Mg, Ag etc.), Potassium dichromate, NaOH, Sodium carbonate, NaCl, NH4OH, HCl, potassium hydrogenphthalate KH(C8H4O4), H2SO4, KOH, sodiumthiosulphate, Potassium permanganate, benzoic acid (b) Explain equilibrium constant expression for the auto-ionization of water. (c) A 0.5382 g sample containing sodium bicarbonate was dissolved and titrated with standard 0.1263 M hydrochloric acid solution, requiring 43.14 mL of hydrochloric acid solution. The reaction is Page 1 of 2 HCO3- + H+ → H2O + CO2 Calculate the percent sodium bicarbonate in the sample. (d) Briefly explain acid-base theories with examples. 4. (a) Calculate the solubility of zinc (II) hydroxide in a solution maintained at (i) pH= 5.3 (ii) pH= 8.2. Ksp = 2.0 x 10-17 (b) Explain the pH titration curve between strong acid and strong base. (c) Define the following terms: (i) Occlusion (ii) Peptization (iii) Wet Digestion (d) Identify types of volumetric method / titrimetry among following given reactions. (i) Ag+(aq) + Cl- → AgCl(s) (ii) 2HCl + Na2CO3 ⎯→ 2 NaCl + CO2 + H2O (iii) Cr2O72- + 6Fe2+ + 14H+ ⎯→ 6Fe3+ + 2Cr3+ + 7H2O (iv) Ca2+ + EDTA4− → [Ca-EDTA]2− (v) H2C2O4. 2H2O + 2NaOH ⎯→ Na2C2O4+ 4H2O (vi) EDTA4− + Mg2+ → [Mg-EDTA]2− (vii) 2KMnO4 + 3H2SO4 + 5(COOH)2 → K2SO4 + 2MnSO4 + 8H2O + 10CO2↑ (viii) Ag+(aq) + Br- → AgBr(s) 5. (a) Calculate the pH of a solution prepared by mixing 1.0 mL of a strong acid solution of pH 3.00 and 2.0 mL of a strong base of pH 10.00. (b) A 1.93 g sample of soil was analyzed and found to contain 3.6 μg Na. What is the concentration of Na in the soil in ppm and in ppb? (c) Identify the oxidizing and reducing agents from the reactants of the following equations: (i) Cr2O72- + U4+ Cr3+ + UO22+ (ii) Ti3+ + 2Fe(CN)64Ti+ + 2Fe(CN)63(iii) Fe3+ + Sn2+ Fe2+ + Sn4+ (iv) H2O2 + Ce4+ O2 + Ce3+ (d) Define the following terms. (i) Chelating Agent (ii) Solubility Product (iii) Gravimetric analysis Note: Check Atomic Weights from Periodic Table. Page 2 of 2