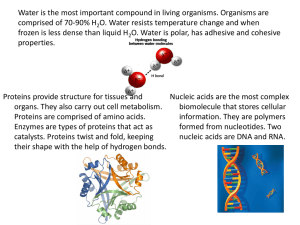
Biomolecules What molecules keep us alive, and how do they do so? Open to the next available page in your journal and construct this chart Title: Biomolecules Biomolecule Monomer Function Carbohydrate Lipid Proteins Nucleic Acids Examples Made up of: Biomolecules • All living organisms require several compounds to continue to live. • We call these compounds biomolecules. All of these biomolecules are organic, which means that they contain carbon. • Carbon has four valence electrons, which means this element forms strong covalent bonds with many other elements. Let’s get started!! Amoeba sisters and Biomolecules Biomolecules • All of our biomolecules are classified into four groups: • • • • Carbohydrates Lipids Proteins Nucleic Acids • Each of these classes have different structures and functions. Biomolecules • Biomolecules are formed by joining many small units together to form a long chain. • This process is called synthesis. Often, a water molecule is removed in the process. • When this happens, we call it dehydration synthesis. Building up polymers Dehydration Synthesis • Creates a polymer from a biomolecules monomers. • In this process, an OH and H are removed (water) during synthesis of a new molecule. 8 Breaking down polymers • Hydrolysis breaks a covalent bond by adding OH and H from a water molecule. Biological molecules 10 Dehydration vs. Hydrolysis Biomolecules • The smallest functioning unit of a biomolecule is a monomer. • “Mono-” means ONE. • Put two monomers together, and you get a dimer. • Di-” means TWO. • Once several monomers are put together, we get a polymer. • “Poly-” means MANY. Carbohydrates • Carbohydrates are biomolecules used for energy and structural support. • Breaking carbohydrates down provides an organism with energy. Carbohydrates • Carbohydrates are made up • Monomer: of carbon, hydrogen and oxygen. • The ratio of these elements is roughly 1 carbon: 2 hydrogen :1 oxygen. C6H12O6 Monosaccharide • Dimer: Disaccharide • Polymer: Polysaccharide Carbohydrates • Carbohydrates are primarily used to provide us with energy. • All monosaccharides and disaccharides end in “-ose”. • Glucose is used as a common energy source for most organisms. Carbohydrates • There are many other types of carbs in nature: • Fructose (fruit sugar) • Lactose (milk sugar) • Sucrose (table sugar) • Ribose/Deoxyribose (important for DNA and RNA) Carbohydrates • Carbohydrates can be bonded to each other through dehydration synthesis. • Remember, that’s when water is lost as two smaller molecules bond to form a larger molecule. Carbohydrates Carbohydrates • When we have excess carbs, we store them as starches, which are polysaccharides. • Starches are long chains of carbs. • Plants also use cellulose (another polysaccharide) for structural support. Carbohydrates • Indicators are chemicals that detect the presence of a certain compound. • Benedict’s solution reacts with MOST mono- and disaccharides. • Sucrose is a notable exception! Carbohydrates • If a detectable carbohydrate is present, then the indicator changes color, based on how many carbs are present. • Green → Yellow → Orange → Red Carbohydrates • Iodine is used to detect starch, since it reacts readily with starch. • This reaction produces a purpleblack coloration. Lipids • Lipids are used for four crucial purposes: • • • • Storing energy Waterproof barriers Chemical messengers Insulation Lipids • Lipids are made up of carbon, hydrogen and oxygen. • The ratio of these elements is roughly 1carbon: 2 hydrogen. Oxygen is present only in trace amounts. • Most common lipids are composed of two different functional groups: • Glycerol, an alcohol with three oxygen groups. • Fatty acids, which are long hydrocarbon chains. Lipids • ALL lipids repel water, due to how hydrophobic they are. This means that they do not bond to water molecules. Lipids • Lipids are grouped by the number of double bonds found in the hydrocarbon chain. • Saturated fats have the maximum number of hydrogen atoms possible, and as such, they have no double bonds. • They tend to be solid at room temperature. Lipids • Unsaturated fats have double bonds. They do NOT have the maximum possible number of hydrogen atoms. • They tend to be liquid at room temperature. • Monounsaturated fats have only ONE double bond. • Polyunsaturated fats have MORE THAN ONE double bond in the hydrocarbon chain. Lipids Monounsaturated Polyunsaturated Lipids • It’s important to note that fats are a specific type of lipid. • Chemically, all fats are triglycerides – they have three fatty acids bonded to one glycerol molecule. Lipids • Steroids are lipids with four rings bonded together. • Steroids are vital as hormones, which are chemical signals used in the body. Lipids • Oily and fatty foods tend to • We can also use ethanol, leave stains upon contact. which dissolves lipids. • This is why we can use brown paper to detect fats. • The dissolved fats are then diluted with water. Since water and lipids don’t mix, the lipids come out of solution. • This creates an emulsion – a milky, cloudy liquid. Protein • Proteins serve many vital functions in the body: • Structural support • Enzymes (Speeding up chemical reactions) • Transport of molecules • Fighting infection • …and many more! Protein • All proteins contain carbon, hydrogen, oxygen and nitrogen. • In addition, sulfur may be present as well. • Monomer: Amino acid • Polymer: Protein or polypeptide • A peptide is a chain of amino acids, so a polypeptide is several chains put together. Protein • ALL amino acids contain an amino or N-group. It contains nitrogen (N). • ALL amino acids also contain a carboxyl or C-group. It contains carbon (C). Protein • However, amino acids also have a variable group or R-group. This differs from one amino acid to the next. • There are 20 standard amino acids, and thus 20 possible R-groups. Protein • Amino acids are bound together through dehydration synthesis. • The C-group of one amino acid binds to the N-group of another. • We call these bonds peptide bonds. Protein • Proteins can also function as hormones. • However, protein hormones tend to have difficulty passing the cell membrane. • As such, many protein hormones have to fit a cellular receptor before they can affect the cell. Protein Production • Proteins have four phases of production: • Primary: Amino acids are bound together. • Secondary: Individual amino acids are bent and molded as needed. • Tertiary: The entire chain of amino acids is bent and molded as needed, forming a sub-unit. • Quaternary: Multiple completed sub-units are fitted together to make a complete protein. Protein Test Indicator • The Biuret test is used to detect protein. • The test relies on a color change to confirm the presence of proteins. If proteins are found, the sample will turn violet. Hydrolysis • Hydrolysis is the reverse process of dehydration synthesis. • In dehydration synthesis, water is lost to create a bigger molecule. • In hydrolysis, water is ADDED, and a bigger molecule is broken down into smaller pieces. • Hydrolysis = hydro and lysis. Hydro means water, and lysis means to break down. Nucleic Acids • Nucleic acids are biomolecules that contain the blueprints for making proteins. Nucleic acids also transmit genetic info to the next generation. • Includes: • DNA • RNA Nucleic Acids • Nucleic acids contain carbon, hydrogen, oxygen, nitrogen, and phosphorus. • Remember the acronym: CHONP! • Monomer: Nucleotides • Polymer: Nucleic Acid • Examples: DNA, RNA Nucleic Acids Monomer- Nucleotide • A nucleotide is made up of three parts: • 5-carbon sugar • Phosphate group • Nitrogenous base Nucleic Acids • The 5-carbon sugar is deoxyribose, in the case of DNA. • However, it is ribose in the case of RNA. • This is how those molecules got their name! Nucleic Acids • As stated earlier, nucleic acids are the blueprints for proteins. Proteins are made from these templates. • Also, DNA can be passed on from parent to child. This allows SOME characteristics to be passed down to offspring. These traits are considered hereditary. • RNA can NOT be passed down to offspring, however!