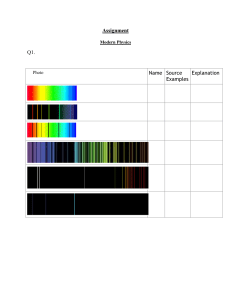
CHAPTER 2.0 ATOMIC STRUCTURE CHAPTER 2.1 BOHR’S ATOMIC MODEL Learning Outcomes a) Describe Bohr’s atomic model b) Explain the existence of energy levels in an atom c) Calculate the energy of an electron using, En = -RH(1/n2) RH = 2.18 x 10-18 J d) Describe the formation of line spectrum of hydrogen atom e) Illustrate the formation of Lyman, Balmer, Paschen, Brackett and Pfund series. f) Calculate the energy change of an electron during transition: ΔE = RH (1/ni2 -1/nf2) , RH = 2.18 x 10-18 J g) Calculate the photon of energy emitted by an electron that produces a particular wavelength during transition: ΔE = hv , v = c/λ C1 ✓ C2 LO 2.1 C4 ✓ ✓ ✓ ✓ ✓ h) Perform calculations involving the Rydberg equation. 1/λ = RH (1/n12 – 1/n22) , RH = 1.097 x 107 m-1 and n1<n2 i) Calculate ionisation energy of hydrogen atom from Lyman series. j) State the limitation of Bohr’s atomic model. k) State the dual nature of electron using de Broglie’s postulate and Heisenberg’s uncertainty principle. C3 ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ ✓ a) Describe Bohr’s atomic model Bohr’s Atomic Postulates In 1913, a theoretical explanation of the emission spectrum of the hydrogen atom was presented by the Danish physicist Niels Bohr. He assumed that: 1. Electrons moves in circular orbits. 2. The energies associated with electron motion in the permitted orbits must be fixed in value, or quantized. LO 2.1 c) Calculate the energy of an electron using, En = -RH(1/n2) RH = 2.18 x 10-18 J 3. The emission of radiation by an energized hydrogen atom when electron dropped from a higher energy orbit to a lower one and give off a quantum of energy (a photon) in the form of light. En = -RH (1/n2) Calculate the energy of an electron using, where RH, the Rydberg constant, has the value 2.18 x 10-18 J. The number n is an integer called the principal quantum number, it has the values n = 1,2,3,.. EXAMPLE 1 Calculate the energy of an electron when it occupies (a) n = 1 , in joules per atom (b) n = 4 in kilojoules per mole SOLUTION (a) En = -RH(1/n2) E1 = - 2.18 x 10-18 (1/12) = - 2.18 x 10 -18 J per atom (b) E4 = -2.18 x 10-18 (1/42) = -1.36 x 10 -19 J per atom = (-1.36 x 10-19) x (6.02 x 1023) J per mole = (-1.36 x 10-19) x (6.02 x 1023) kJ per mole 1000 = -81.9 kJ mol-1 LO 2.1 f) Calculate the energy change of an electron during transition: ΔE = RH (1/ni2 -1/nf2) , RH = 2.18 x 10-18 J 4. Radiant energy (in the form of a photon) is emitted when the electron moves from a higher-energy state (Ei) to a lower-energy state (Ef). The difference in energy between energy levels is ΔE = Ef - Ei Ef = – RH (1/nf2) and Ei = –RH (1/ni2) Where ni = initial orbit nf = final orbit Therefore, ΔE = –RH (1/nf2) – ( –RH (1/ni2)) = RH (1/ni2 – 1/nf2) Because this transition results in the emission of a photon of frequency, v and energy, hv it can be written : ΔE = hv = RH (1/ni2 – 1/nf2) EXAMPLE 2 Calculate the energy liberated when an electron from the fifth energy level falls to the second energy level in a hydrogen atom. SOLUTION : ΔE = RH (1/ni2 – 1/nf2) = (2.18 x 10-18) x ( 1/52 – 1/22) = -4.58 x 10-19J The negative value of ΔE shows that energy is released. LO 2.1 (g) Calculate the photon of energy emitted by an electron that produces a particular wavelength during transition: ΔE = hv , v = c/λ EXAMPLE 3 Calculate the photon energy emitted by an electron that produces a wavelength of 4.342 x 10-7 m SOLUTION : v = c/λ = 3.00 x 108 ms-1/ 4.342 x 10-7 m = 6.91 x 1014 s-1 ΔE = hv = 6.63 x 10-34 Js x 6.91 x 1014s-1 = 4.58 x 10-19J Exercise 1 : a)The energy (in J) of an electron has when it occupies a level equivalent to the quantum number of n = 3 and n = 4. b)The energy (in kJ/mol) of photon emitted when one mole of electron drops from the 4th to the 3rd energy level. c) The frequency (in s-1) and wavelength (in nm) of this photon. ANS: a) E3 = – 2.42 x 10-19 J , E4 = – 1.36 x 10-19 J b) – 63.8 kJ/mol c) v = 1.60 x 1014 s-1 λ = 1875 nm Exercise 2 Calculate the wavelength (in nm) of a photon emitted by a hydrogen atom when its electron drops from the n = 5 state to the n = 3 state. ANS : λ = 1283 nm Exercise 3 An electron in the hydrogen atom makes a transition from an energy of ni to n = 2 state. If the photon emitted has a wavelength of 434 nm, what is the value of ni ? Exercise 4 Use the Rydberg equation to calculate the wavelength (in nm) of the forth line in the Balmer series of H spectrum . ANS : λ = 410.2 nm Line Spectra and Continuous Spectra When white light from an incandescent lamp is passed through a prism, it produces a continuous spectrum, or rainbow colours . The different colours of light represent different wavelengths. All wavelengths are present in a continuous spectrum. White light is simply a combination of all the various colours. If the light from a gas discharge tube containing a particular element is passed through a prism, only narrow coloured line are observed. Each line corresponds to light of a particular wavelength. The pattern of emitted by an element is called its line spectrum. The line spectrum of hydrogen is fairly simple; it consists of four lines in the visible portion of the electromagnetic spectrum. The emission spectrum of hydrogen includes a wide range of wavelengths from the infrared to the ultraviolet. They are named after their discoverers. The Balmer series was particularly easy to study because a number of its line falls in the visible range. LO 2.1 (d) Describe the formation of line spectrum of hydrogen atom LO 2.1 (e) Illustrate the formation of Lyman, Balmer, Paschen, Brackett and Pfund series. Series nf ni Spectrum Region Lyman 1 2,3,4, .. Ultraviolet Balmer 2 3,4,5, .. Visible Ultraviolet Paschen 3 4,5,6, .. Infrared Brackett 4 5,6,7, .. Infrared Pfund 6,7,8, .. Infrared Figure : Shows the energy levels in the hydrogen atom and the various emission series. 5 and Table : The various series in atomic hydrogen emission spectrum. LO 2.1 (f) Perform calculations involving the Rydberg equation. 1/λ = RH (1/n12 – 1/n22) , RH = 1.097 x 107 m-1 and n1<n2 This equation solve problems based on transitions of the electrons in the Lyman to Pfund Series. 1 / = RH (1/ni2 – 1/nf2) where ni < nf RH = 1.097 x 107 m-1 Exercise 5 Explain how the hydrogen spectrum formed ANSWER Example 4 : Calculate the wavelengths of the first line and the onset of the continum limit for the Lyman series for hydrogen. First line : 1/ = RH (1/ni2 – 1/nf2) = 1.097 x 107 (1/12 – 1/22) = 1.21 x 10-7m-1 Onset of the continum limit : 1/ = RH (1/ni2 – 1/nf2) = 1.097 x 107 (1/12 – 1/) = 1.097 x 107 (1/12 – 0) = 9.12 x 10-8 m-1 Exercise 6 Calculate (i) the wavelength in nanometer and (ii) frequency of the line in the Balmer series that results from the transition n=4 to n=2. SOLUTION : (i) 1/λ = RH (1/22-1/42) =1.097 x 107 (1/4-1/16) = 2.057 x 10 6 λ = 4.86 x 10-7 m (ii) v = c/λ = 3.00 x 108 4.86 x 10-7 = 6.17 x 10 14 s-1 LO 2.1 (i) : Calculate ionisation energy of hydrogen atom from Lyman series. Ionization Energy Ionization energy is the minimum energy required to remove one mole of electron from one mole of gaseous atom or ion. E (g) E+ (g) + e-(g) where E represents an element. All ionization energies are positive because energy always required to remove an electron. Ionization energy for hydrogen : ni = 1, nf = ΔE= 2.18 x 10-18 (1/12 – 1/) = 2.18 x 10-18 (1/12 – 0) = 2.18 x 10-18 J.e-1 x 6.023 x 1023 e/mole = 1.313 x 10 6 kJ mole-1 LO 2.1 (j) : State the limitation of Bohr’s atomic model. The Weakness of Bohr’s Atomic Postulates Bohr’s approach did not account for the emission spectra of atom containing more than one electron. This approach also did not explain why extra lines appear in the hydrogen emission spectrum when magnetic field is applied. Another problem is that electrons are wavelike. We cannot define the precise location of a wave because a wave extends in space. LO 2.1 (k) : State the dual nature of electron using de Broglie’s postulate and Heisenberg’s uncertainty principle. The Dual Nature of the Electron Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance?. For a decade there is no logical explanation. In 1924, Louis de Broglie provided the solution for this puzzle. If light wave can behave like a stream of particles (photon) then, perhaps particles such as electron can possessed wave properties. De Broglie reasoning led to the conclusion that waves can behave like particles and can exhibit wavelike properties. It was related by expression where; = wavelength of moving particles = h m h = Planck’s constant m = mass of particles = velocity of moving particles Example 5 : Calculate the wavelength of the particles in the following of two cases. a) The fastest serve in tennis is about 62 ms-1. Calculate the wavelength associated with 6.0 x 10 kg tennis ball traveling at this velocity. b) Calculate the wavelength associated with an electron moving at 62 ms-1. ANSWER a) From = = h m 6.63 x10 34 Js 6.0x10 2 kgx 62 ms 1 = 1.8 x 10-34m. This is exceeding small wavelength. For this reason, the wave properties of such a tennis ball cannot be detected by any existing measuring device. b) From = = h m 6.63 x10 34 Js 9.1095 x10 31 kgx 62 ms 1 = 1.20 x 10-5m or 1.20 x 104nm (in infrared region) Exercise 7 : What is the de Broglie wavelength (in meters) of a small car with a mass of 1150kg traveling at a speed of 24.6ms-1? (Ans: 2.34 x 10-38m) Calculate the wave length of the “particle” in the following two cases: a) The fastest serve in tennis is about 140 miles per hour or 62 m/s. Calculate the wavelength associated with a 6.0 x 10-2 kg tennis ball traveling at this velocity. b) Calculate the wavelength associated with an electron moving the same velocity. Mass of electron = 9.1095 x 10-31 kg