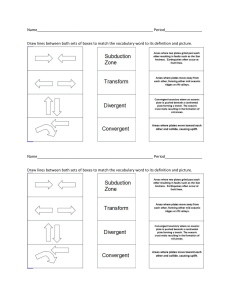
Lab Lesson Plan McKenzie Funk Class: Physical Science Grade: Freshmen Mystery Solutions Lab! NGSS HS-PS13. Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. More Specifically: Planning and Carrying Out Investigations Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models. Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly. During part of this lab the students brainstorm to come up with ways to identify the solutions in the bottle. This is the students planning and then conducting an experiment, they also have to work together to identify and match the solutions. Student Learning Objective: At the end of the lesson the student will be able to identify with 75% accuracy which sections of the lab and lab procedure match with each step in the scientific method. Rationale: This is a great lesson to start to introduce the concept of the scientific method. This is something that that will have to use both consciously and unconsciously for not only the rest of the school year, but the rest of their lives. It also helps give them the confidence to both work with a partner and develop a plan and put it into effect. This will also ideally be their first time in full lab setting after a safety module, so this gives them a chance to put that module into effect and it will be something they use all year. Safety-taken from the original lab: The chemicals in this laboratory activity are considered safe. All food-grade items that have been brought into the lab are considered laboratory chemicals and are for lab use only. Do not taste or ingest any material in the lab and do not remove any remaining food items after they have been used in the lab. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Wash hands thoroughly with soap and water before leaving the laboratory. Follow all laboratory safety guidelines. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Materials (for a class of 30 working in pairs- adjust as needed): Aluminum potassium sulfate solution, 2% 200mL Sodium Bicarbonate (baking soda) solution (mixed with water), 2% 200mL Vinegar white, 200mL 2% solution of citric acid, 100mL Empty Cassette boxes, 30 Paper Towels- 1 per student Acetate sheets-cut up into squares-90 Permanent marker Pipets, beral-type, jumbo 90 Pipets, thin stemmed, 90 Plastic cups, wide mouth, 3 Rubber bands, 15 Scissors Paper Copies of the worksheets- both- for everyone Pre-Class Preparation-taken from the original Lab, which is included: Cut the stems off the Jumbo pipets and then cut the bulbs in half. Push three halves, open-side up, sideby-side, into each cassette box. These will act as holding cups for the thin stem pipet bulbs (see Figure 1). 2. Cut off all but 1 cm of the stem off each thin-stem pipet. 3. Label 15 of the thin-stem pipet bulbs “A,” 15 of them “B,” 15 of them “C,” 15 of them “1,” 15 of them “2,” and 15 of them “3.” 4. Place 200 mL of alum solution (2%) in a wide mouth cup labeled “A, 2.” 5. Place 200 mL of baking soda solution (2%) in a cup labeled “B, 3.” Place 200 mL of white vinegar in a cup labeled “C, 1.” 6. Pick up two of the bulbs labeled “A” (one in each hand), squeeze them and then draw up half a bulbfull of the solution from cup “A, 2.” Place these bulbs directly into the first holding cups in the first two cassette boxes. Repeat with two more “A” bulbs, placing them in the third and fourth cassette boxes. Continue until all fifteen “A” bulbs are done (see Figure 1). 7. Pick up two of the bulbs labeled “B” (one in each hand), squeeze them and then draw up half a bulbfull of the solution in cup “B, 3.” Place them directly into the second holding cups in the first two cassette boxes, just along side the “A” bulbs. Repeat until all fifteen “B” bulbs are done. 8. Repeat this procedure with all the “C” bulbs. Set aside these fifteen completed cassette boxes. 9. Pick up two of the bulbs labeled “1” and then draw up half a bulb-full of the solution from cup “C, 1.” Place them directly into the first holding cups in the next two cassette boxes. Repeat until all fifteen “1” bulbs are done. 10. Repeat step 9 with all the “2” bulbs using solution “A, 2.” 11. Repeat step 9 with all the “3” bulbs using solution “B, 3.” 12. Cut the overhead transparencies into rectangular sheets approximately 3 cm x 5 cm. 13. With a permanent marker, place a dark spot in opposite corners of each sheet, and then slide them into each of the thirty cassette boxes, wedged in behind the holding cups (See Figure 1). Note: The dark spots in the corners just make it easy to find the sheets should they fall onto the floor, and it also helps confirm that the students are remembering to turn the sheets back in when the boxes are being returned. 14. Close the boxes and rubber band them together in pairs—a numbered box (1, 2, 3) paired up with a lettered (A, B, C) box -Mix up the numbers and letters so that they are more randomized, and less likely to cheat/copy. Beginning (10-15 Minutes)Start the class with a review of the scientific method and the steps and go over the procedure. The students should have been given the procedure to read over the day before. Also go over safety with the students. Stress that you cannot eat the chemicals in a lab. Have them break off into pairs, they are allowed to choose as they will not be physically speaking. Number the groups off 1-15 (again, based on a class of 30, adjust as needed). Stress that they are only allowed to communicate through notes- any other form of communication is not allowed and if caught talking they will receive a 1-point deduction each time. Middle (30-35 minutes)Procedure-taken from the original lab1. With students working in pairs, assign each pair a group number (1 through 15). 2. Hand out the cassette box pairs, one set-up to each pair of students. Tell them to look at the boxes but do not open them up yet. 3. Hand out the Mystery Solutions Lab Worksheets, one to each pair. 4. Explain the objective of the lab is to determine which solutions in the numbered box match the corresponding solutions in the lettered box. 5. Allow students a few minutes to brainstorm different ways to solve the problem, writing down their ideas in the space provided on the worksheet. 6. As a class, have the students discuss the ideas they came up with; invariably a student will offer the approach of mixing the solutions together to see what happens. Discuss the merits of this approach and instruct them all to give it a try. Show them how to use the cut-off pipets to squeeze out drops and mix them on the acetate sheet, and how to avoid contamination—never placing the tip of a pipet into a drop of a different solution. 7. Explain to the students one final restriction—they are not to talk with their partners, nor have any visual contact. Their only means of communicating their findings is by writing, or “faxing.” 8. Have the students separate from their partners (across the room) or have them at least sit squarely back-to-back. If they are across the room, then you and/or a designated helper must serve as the “fax machine” carrying their messages on the lab sheet back and forth between them. If they are back-toback, simply have them pass the sheet behind them. You may need to re-emphasize with some groups that the goal is to get the correct matchups for the solutions. Collect their results on a separate sheet as they finish. 9. Have them dispose of the chemicals as outlined in the Disposal section of the procedure. (on the worksheet) End- Rest of class time- 5-10 minutesDiscuss as a class the pros and cons of this communication style- what worked and what did not? Start to discuss what steps in the scientific method they can easily identify- write it on the board for them to see easily. If they did not get done with the reasoning section of the worksheet, stress that it is due at the start of class time tomorrow for a participation grade. They will not be penalized for if they got the solutions correctly matched, but on participation and their reasoning. Assessment-formal: The worksheet from the lab- mostly for participation. Points will be deducted if their reasoning is not sound, if they did not follow proper lab safety, or if they talked/communicated in a way that was not just written notes. The second worksheet about the scientific method will be taken for a grade. It will be due start of class the next day, and they will have the opportunity to correct their mistakes to get credit back. SOURCES “HS-PS1-3 Matter and Its Interactions | Next Generation Science Standards.” Nextgenscience.org, 2020, www.nextgenscience.org/pe/hs-ps1-3-matter-andits-interactions. Accessed 10 Dec. 2020. Mystery Solutions Lab Scientific Method Inquiry Lab Activities.