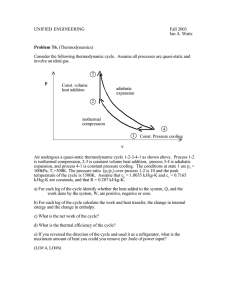
amics Thermod Thermodynamics is the branch of physics in which we study the interrelationshipbetween the heat energy and the mechanical energy. A substance (or a body) bounded by a surface which is affected by heat is called a thermodynamic system. The properties of a system which determines the thermodynamic state of that system are called the thermodynamic variables. The thermodynamic state of a gaseous system is expressed in terms of the sariables pressure P, volume V and temperature T.Thus, the thermodynamic variables of a gaseous system are P. V and T. The equation relating the thermodynamic variables of a system is called equation of state of that system. For I inole of an ideal gas, the equation of a state is PV = RT . Out of the thermodynamic variables P, V, and T of a gaseous system. and the third variable is dependent. variables are independent Different types of thermodynamical processes: (i) Isobaric process: (A) It is a thermodynamic process in sshich pressure is kept constant. (B) The equation of state for the process is: (VT) = constant. (C) In isobaric compression, temperature decreases and internal energy flows out in the form of heat energy, SShilein isobaric expansion, temperature increases and heat flows into the system. (D) The amount of heat energy AQ transferred is given by: AQ = gC? AT (g = number of moles) (E) In. the adjoining figure, graphs I and Il represent isobaric expansion and compression p respectively. (F) The slope of the isobaric curve on a P-V diagram is zero. (G) Isobaric expansion of the volume of a gas is given by: Vt = Vo(l + 'fit) where = (1/27 3) per cc = coeff. of volume expansion. (ii) Isochoric (Isometric) process: (A) It is a thermodynamic process in 'Whichthe volume of the system is kept constant. (B) The equation of state for the process is: (PT) constant. (C) For increasing (decreasing) the pressure of a gas at constant volume, its temperature must be increased (decreased) by adding (taking Out) heat energy into (from) the system. gCvAT. (D) For isochoric process: (E) In the adjoining figure, graphs I and Il represent isometric decrease in pressure at volume VI and Isometnc increase in pressure at volume V: respectively. c (F) The slope of the isometric curve is infinite on a P-V diagram. (G) Isometric expansion of the pressure of a gas is given by: +7pt) where (1/273) per oc = 0.0037 per oc = coefficient of pressure expansion. (iii) Isothermal process: (A) It is a thermoclynamical process in which the temperature of the system remains constant. (B) The equation of state for the process is: PV = constant. (C) During isothermal expansion of a gas, its volume increases while pressure decreases, while in isothermal compression, the volume decreases while pressure increases. (D) The isothermal curve on P-V diagram is like a hyperbola and is shown in the adjoining figure. (e) The slope of the isothermal curve at any point M is given by:— — dv Initial state Final state v Volume (V) It is negative arid is equal in magnitude to the ratio of P and V at that point. (f) kn an isothermal process, heat may go into or may come out of the system to maintain constant temperature. (iv) Adiabatic process: (A) It is a process in which heat exchanged by the system to or from the surroundings Adiabauc is zero, i.e. , AQ = 0 (B) The equation of state for this process is: PVY= constt. where y is the ratio of specific heats of the gas. (C) The adiabatic curve on the P-V diagram is shown in the adjoining figure. The slope of the curve at any point is given by Slope ofadiabatic curve is more in magnitude in comparison to the slope of the isothermal curve. p pa Isothermal Adiabatic Piso Piso 5Ääi-a-tÄti0 'c Isothermal (D) When a gas expands from some initial volume to final volume, then final pressure is less for adiabatic expansion. But, when a gas compresses from some initial volume to a final volume, then the final pressure is more in case of adiabatic compression. (e) Equation of state for the adiabatic process can be any of the following three types: (Ill) p i -YYY= cosnstt. (Il) TV -I = constt. (I) PVY= constt. (F) In adiabatic expansion, temperature decreases while in adiabatic compression, temperature in creases. (G) Isothermal and adiabatic bulk modulus of a gas are given by: = P and Bisothermal = Badiabatic (v) Cyclic procuss : (A) inial and final position of system is sam (B) change voleme is zero thus work done will be zero First law of thermodynamics: (i) First law of thermodynamics is equivalent to law of conservation of energy. (ii) When heat energy is supplied to a gas. two things may occur: (A) the internal energy of the gas may change. (B) the gas may do external work by expanding. (iii) According to this law if heat AQ is added to a system then it will show up either as a change in internal energy AU of the system andlor as work AW performed by the system, i.e., AQ=AU+AW or First law of thermod namicsa S.N0. 1. process Cyclic 2. Isochoric 3. Isothemal 4. Adiabatic lied to different rocesses: 0 AW Area of the closed curve gcvAT gRT loge -z 0 P(V2 —VI) = HR(T2 —T!) 5, Isobaric gcpAT gcv AT For I mole gas, work done in an isothermal process. is W = RT loge -z = RT loge In-I in adiabatic process, adiabatic law is obeyed according to which PVT= constant = cosntant or P' -YTY= constant. For 1 mole of gas, work done in an adiabatic process is — 7-1 7-1 Efficiency of the engine = WorkobtainedbytheengineinonecycleJV Heat absorbed Q A Carnot engine is not realisable in practice. The working substance in a Carnot engine is an ideal gas. Efficiency of Carnot engine n I where Tl = temperature of source, T2 temperature of sink, = adiabatic expansion ratio and 7 = ratio of the two specific heats.