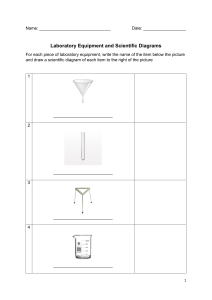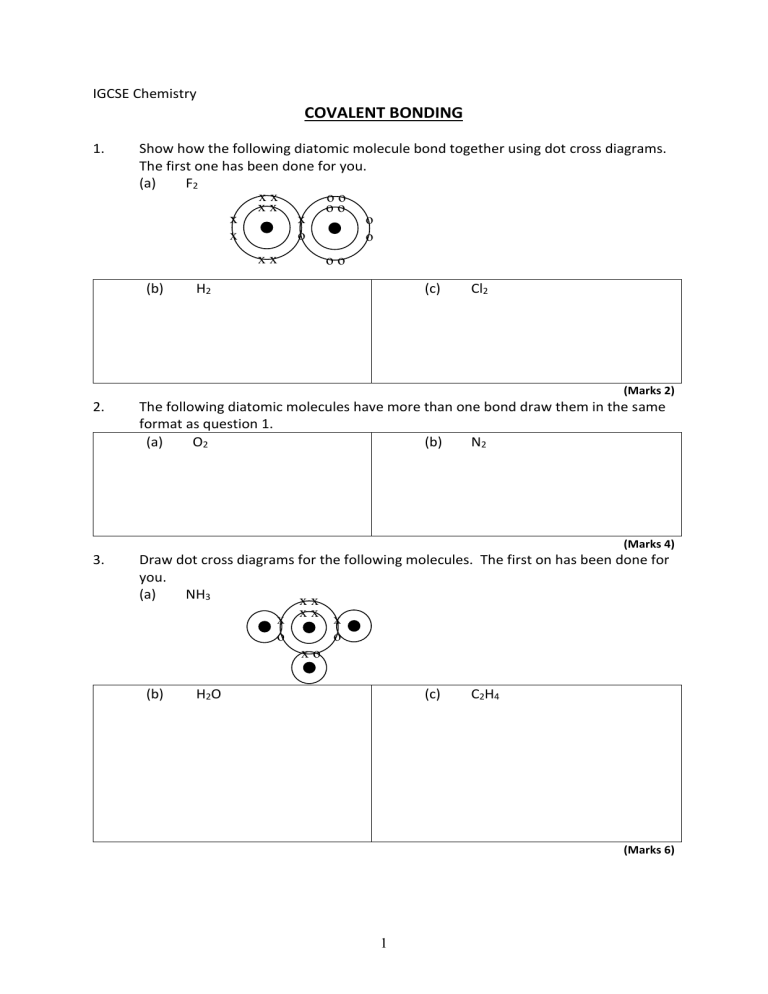
IGCSE Chemistry COVALENT BONDING 1. Show how the following diatomic molecule bond together using dot cross diagrams. The first one has been done for you. (a) F2 xx oo xx oo x x o x o o xx (b) oo H2 (c) Cl2 (Marks 2) 2. The following diatomic molecules have more than one bond draw them in the same format as question 1. (a) O2 (b) N2 3. Draw dot cross diagrams for the following molecules. The first on has been done for you. (a) NH3 xx xx x x o o xo (Marks 4) (b) H2O (c) C2H4 (Marks 6) 1 4. Draw dot cross diagrams for the following molecules. (a) Methanol H H C (CH3OH) OH H (Marks 8) 2