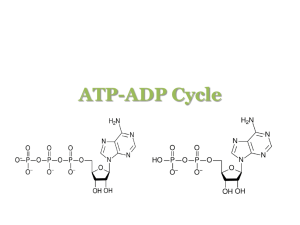
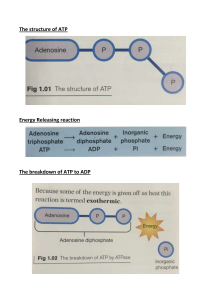
Name: __________________________________________________ Period: __________ Date: _________________ ACTIVITY: What is “Energy” in Living Systems? Investigating Chemical Energy Background Energy within the cell exists in the form of chemical energy. A source of this chemical energy is a compound called adenosine triphosphate (ATP). ATP, when changed to a compound called adenosine diphosphate (ADP), releases energy for biological work in a cell. ADP can be changed back to ATP, but this chemical reaction requires energy. Where does all this energy come from?? Remember that energy is “stored” in the bonds of biomolecules like carbohydrates and lipids. Food such as glucose contains much energy! To learn more about chemical energy you will: (a) use paper models to construct molecules of ATP and ADP, (b) determine similarities and differences between ATP and ADP, (c) illustrate energy release when ATP is changed to ADP, and (d) study the ATP-ADP cycle. Procedure Part A. The Chemical Structure of Adenosine Triphosphate (ATP) ATP is made up of smaller molecules of subunits – ribose, adenine, and phosphoric acid (or phosphate groups). I. Ribose Molecule H HO C OH H O C H H C C H Examine the structural formula of ribose. Ribose is a carbohydrate, or a sugar. It is different from glucose in one very important way. Glucose has six atoms of carbon in each molecule, and has the chemical formula C6H12O6 . Keep this in mind when answering the following questions. C Based on its name, how do we know that ribose is a carbohydrate, or sugar? H OH OH The molecular formula of glucose is C6H12O6. Notice that the number of atoms of each element present is written as a subscript. Now you try it! Looking at the ribose molecule above, what is the molecular formula of ribose? (Hint: Count the # of atoms of each!). Ribose: C___H___O___ How does the number of hydrogen atoms compare to the number of oxygen atoms in ribose? In observing the molecular formulas of both glucose & ribose, what difference do you observe about the two molecules? II. Adenine Molecule III. Phosphoric Acid Molecule Examine the structural formula of adenine: Examine the structural formula phosphoric acid. Phosphoric acid is much like the phosphate groups in ATP. NOTE: The letter “P” represents the element phosphorus. H N H O C N H C N C O P O H H O C C H N N H H What is the molecular formula of phosphoric acid? What is the molecular formula of adenine? H___ P O___ C___H___N___ What element is in adenine that is not in carbohydrates (such as ribose)? What element is in ribose (a carb) that is not in adenine? IV. Constructing an ATP Molecule An ATP molecule is made up of 1 ribose molecule, 1 adenine molecule, and 3 phosphate groups joined. What does the prefix tri- in phosphate mean? ___________________________ Adenosine is a molecule that is made up of two separate molecules we just discussed above. Part of adenosine is composed of a ribose sugar (where the letters “os” of adenosine come from). What is the other molecule that makes up adenosine? How do you know? When your team gets to this point, please check in with your instructor for a stamp! Cut out the models of adenine, ribose and phosphoric acid included. Cut along solid lines only. Attempt to join the adenine and ribose molecules much as you would pieces of a puzzle. You will see that they really do not fit! What end must first be removed from each molecule in order for adenine and ribose to fit together? (Hint: what could we remove by cutting on the dotted lines??) Remove the H (hydrogen) from adenine and the O-H (oxygen & hydrogen) from ribose. The adenine and ribose molecules can now be chemically joined. New points of attachment or chemical bonds are formed. Join the parts that were removed like a puzzle. What molecule is formed from the parts that are removed? Examine the phosphate molecules. Attach one of the three phosphates to the ribose molecule by removing a hydrogen atom from the phosphate molecule. Attach the remaining phosphate molecules one at a time to the phosphate group already attached to the ribose. Again, what molecule is formed from the parts removed in making these connections? You have now built an ATP molecule! List the five “building blocks” that are needed to form one ATP molecule. (Hint: what 5 pieces are in front of you?) 1) _________________________ 3) _________________________ 2) _________________________ 4) _________________________ 5) _________________________ What is required for the chemical combination of these parts? (Hint: see the lab introduction) When your team gets to this point, please check in with your instructor for a stamp! Part B. Gaining Energy from ATP as It Changes to ADP Remove one phosphate group from the end of your ATP model. How many phosphate groups are still attached to the original molecule? __________ This new compound with one fewer phosphate groups than before is called adenosine diphosphate (ADP). What does the prefix di- mean? List the four “building blocks” that are needed to form one ADP molecule: 1) _________________________ 3) _________________________ 2) _________________________ 4) _________________________ Explain how an ATP molecule is changed to an ADP molecule. What is released when ATP is changed to ADP? ________________________ So far, we have seen that ATP can be changed to ADP with energy released. This change can be illustrated in a chemical equation for the reaction. For example, this change might be written as follows: ATP ADP + Phosphoric Acid + E What might the letter “E” in the above equation be an abbreviation for? __________________________ Part C. Transforming ADP to ATP ATP can be formed within living organisms if the correct raw materials are available. These raw materials are ADP, phosphoric acid, and energy. We can again use models to help show how ATP is formed. Construct an ADP molecule with your model. Attach an additional phosphate molecule to the ADP model. This combination forms an ATP molecule. Energy is needed to change ADP back to ATP. This change can be illustrated in a chemical equation for the reaction: ADP + Phosphoric Acid + E ATP What might the letter “E” in the above equation be an abbreviation for? __________________________ When your team gets to this point, please check in with your instructor for a stamp!