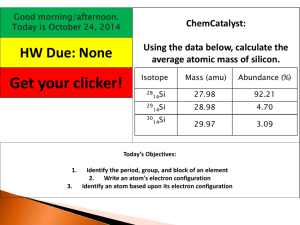
Module 4.1: The Mole Concept and Molar Mass Objectives • to be able to define atomic mass unit • to calculate the average atomic mass of elements • to calculate the mass of a given number of moles of an element or compound, or vice versa • to calculate the mass of a given number of particles of an element or compound, or vice versa Atomic Mass It is measured in atomic mass unit (amu), a relative unit based on the value of exactly 12 for the C-12 isotope. 1 amu = 1/12 the mass of C-12 atom Atomic Mass The atomic mass of Cu-63 is 62.93 amu. This means that relative to C-12, one atom of Cu63 is 62.93/12 or 5.2 times the mass of C-12 atom Exercises One atom of Se-77 is 6.410 times as heavy as an atom of C-12. What is the atomic mass of Se-77? Answers One atom of Se-77 is 6.410 times as heavy as an atom of C-12. What is the atomic mass of Se-77? 6.410 x 12 amu = 77 amu Average Atomic Mass The average atomic mass takes into account the different isotopes of an element and their relative abundances. It is not a simple average but a weighted average. Average Atomic Mass Isotopes of elements occur in different abundances. Some are more abundant than others. Carbon has two isotopes. The natural abundance of C-12 is 98.90% while that of C-13 is 1.10%. The atomic mass of C13 is 13.00335 amu, while C-12 is 12 Average Atomic Mass Average atomic mass of Carbon = (atomic mass C-12)(% abundance C-12) + (atomic mass C-13)(% abundance C-13) = (12.00 amu)(0.9890) + (13.00335 amu)(0.0110) = 12.01 amu Exercises Copper has two stable isotopes with the following masses and abundances: Cu-63 (62.93 amu, 69.09% abundance) and Cu-65 (64.9278 amu, 30.91% abundance). Calculate the average atomic mass of copper. Answers Copper has two stable isotopes with the following masses and abundances: Cu-63 (62.93 amu, 69.09% abundance) and Cu-65 (64.9278 amu, 30.91% abundance). Calculate the average atomic mass of copper. Ans: 63.55 amu Exercises An element consists of an isotope with mass of 10.0129 amu and 19.91% abundance, and another isotope with mass of 11.0093 amu and 80.09% abundance. Calculate the average atomic mass of the element and identify what element is stated. Answers An element consists of an isotope with mass of 10.0129 amu and 19.91% abundance, and another isotope with mass of 11.0093 amu and 80.09% abundance. Calculate the average atomic mass of the element and identify what element is stated. Ans: 10.81 amu, Boron Average Molecular Mass The molecular mass is the sum of the average atomic masses of the atoms in the molecule. It is expressed in atomic mass unit, and is numerically equal to the molar mass (grams per mole) of the molecule. Average Formula Mass The formula mass is the sum of the atomic masses of the atoms in the ionic compound. It is also expressed in atomic mass unit, and is also numerically equal to the molar mass (grams per mole) of the substance. Avogadro's Number The mole (mol) is defined as the amount of substance containing the same number of particles as there are atoms in exactly 12g of C-12 isotope. Avogadro's Number One mole of substance is equivalent to the Avogadro's number of particles: 23 10 6.022 x particles/mole Molar Mass The molar mass of a compound (molecular or ionic) is the mass in grams of one mole of a substance. It is numerically equal to the molecular mass of formula mass. Exercises One mole of C-12 has a mass of exactly 12 grams and one mole of C-12 has Avogadro's number of atoms. Calculate the mass of one atom of C-12 in grams, then calculate the mass in grams of 1 amu. Answers One mole of C-12 has a mass of exactly 12 grams and one mole of C-12 has Avogadro's number of atoms. Calculate the mass of one atom of C-12 in grams, then calculate the mass in grams of 1 amu. -23 -24 Ans: 1.993 x 10 g; 1.661 x 10 g Conversions ÷ Particles 6.022 x 1023 × ÷ Moles Molar Mass × Mass Short Assessment 1. How much heavier is an atom of Br relative to an atom of C? 2. Which element has an average atomic mass that is about ten times that of fluorine? Short Assessment Element A consists of isotope A-6 with natural abundance of 7.5% and a mass of 6.0151 amu, and isotope A-7 with natural abundance 92.5 and mass of 7.0160 amu. 3. Calculate the average atomic mass of Element A. 4. Identify Element A. Short Assessment Naphthalene as a molecular formula of C8H10. 5. What is the molecular mass of naphthalene? 6. What is naphthalene? the molar mass of Short Assessment 7. Which will have a higher molar mass: 0.500 mol zinc, or 0.250 mol lead? 8. What is the molar mass of lithium carbonate? Short Assessment 9. A bottle of Calcium supplements in tablet form contains 268 g Ca. How many atoms are present in 268 g Calcium? 10. How many moles of Copper are there in 875 g Cu? Short Assessment You have 10 minutes to answer. Answers 1. 6.653 times 2. Osmium, Os 3. 6.94 amu 4. Lithium, Li 5.106.16 amu 6. 106.16 g/mol 7. 0.250 mol Pb 8. 73.89 9. 4.03 x 1024 atoms 10. 13.8 mol