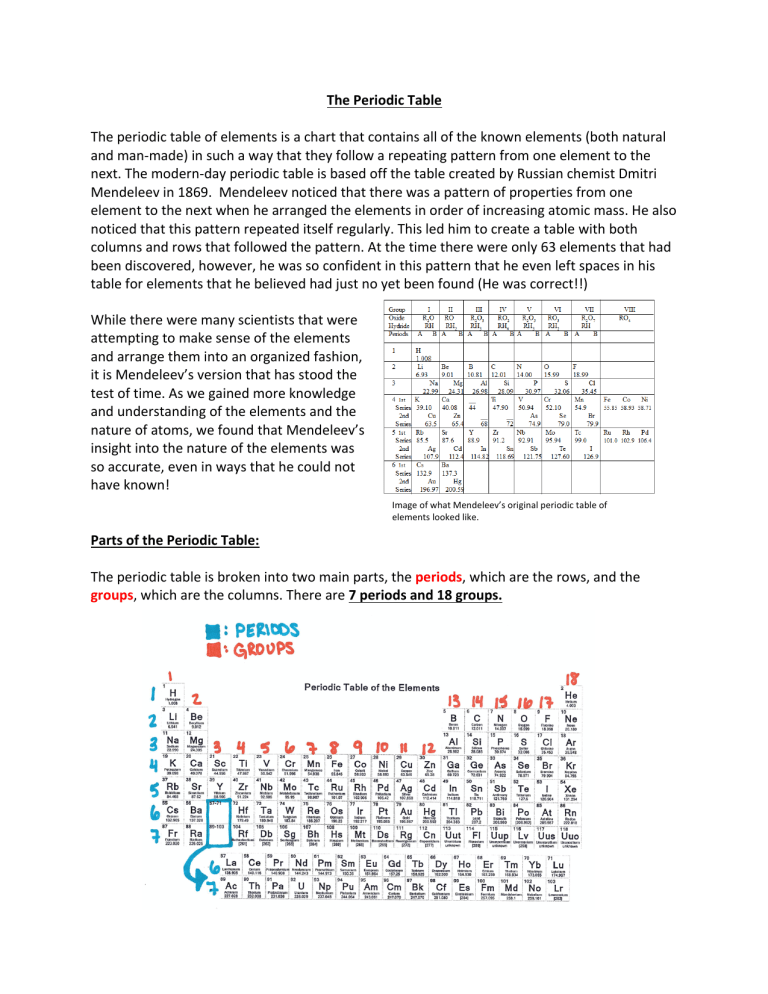
The Periodic Table The periodic table of elements is a chart that contains all of the known elements (both natural and man-made) in such a way that they follow a repeating pattern from one element to the next. The modern-day periodic table is based off the table created by Russian chemist Dmitri Mendeleev in 1869. Mendeleev noticed that there was a pattern of properties from one element to the next when he arranged the elements in order of increasing atomic mass. He also noticed that this pattern repeated itself regularly. This led him to create a table with both columns and rows that followed the pattern. At the time there were only 63 elements that had been discovered, however, he was so confident in this pattern that he even left spaces in his table for elements that he believed had just no yet been found (He was correct!!) While there were many scientists that were attempting to make sense of the elements and arrange them into an organized fashion, it is Mendeleev’s version that has stood the test of time. As we gained more knowledge and understanding of the elements and the nature of atoms, we found that Mendeleev’s insight into the nature of the elements was so accurate, even in ways that he could not have known! Image of what Mendeleev’s original periodic table of elements looked like. Parts of the Periodic Table: The periodic table is broken into two main parts, the periods, which are the rows, and the groups, which are the columns. There are 7 periods and 18 groups. Although it may look like there are nine rows, the two at the bottom (lanthanides and actinides) actually fit into periods 6 and 7. If they were kept within the table it would look like the table below. Types of elements: Within the periodic table there are three different types of elements. These are the metals, nonmetals, and metalloids. Nonmetals are typically liquids or gasses at room temperature and are poor conductors of electricity. Life on Earth depends mainly on nonmetals, carbon, hydrogen, oxygen, and nitrogen leading as far as the most important elements to all living creatures. Metals make up the majority of the table; are typically solids at room temperature and are generally good at conducting electricity. Metalloids fall along the zigzag line on the periodic table that separates the metals from the nonmetals. Depending on the conditions they are under, metalloids will behave either like a metal or a nonmetal. Generally, they are solid, brittle, and good conductors. The most common metalloids is silicone, which is why it is often used in semiconductors and computer chips. Notice that hydrogen is the only nonmetal on the left-hand side of the periodic table. For each of the elements outlined elements in the image below, state what type of element it falls under. 1: ___________________ 5: ___________________ 2: ___________________ 6: ___________________ 3: ___________________ 7: ___________________ 4: ___________________ 8: ___________________ Groups of the periodic table: As mentioned before, the columns are called groups. The elements in each group are have similar properties and characteristics. Group 1, the alkali metals, are the elements in the first column, excluding hydrogen, so from lithium down to francium. These elements all have one electron on their outer shell, which they would like to lose, and they are the most reactive metals on the periodic table Group 2, the alkaline metals, are all the elements in the second column. These elements all have two electrons on their outer shell, which they would like to lose, they are still rather reactive, but less than the alkali metals. Groups 3-12 are called the transitional metals. These elements can have anywhere from 1-3 electrons on their outer shell, which they would like to lose, and there is no specific pattern they are guaranteed to follow. These elements are not as reactive as groups 1 or 2. Groups 13 – 16, named after the elements that are at the top of the column (for example, group 13 is called the boron group). The boron group has three electrons it would like to lose. It is also the beginning of the change from metals to metalloids and nonmetals. The carbon group has four electrons on the outer shell. Carbon would like to gain 4 more electrons, while the remaining in the group generally want to lose them. The nitrogen group elements all have five electrons on the outer shell. The nonmetals and metalloids in the group want to gain 3 electrons and the metals want to lose their 5 electrons. Finally, the oxygen group elements all have six electrons in their outer shell. The nonmetals and one metalloid in the group all want to gain 2 more electrons. The metals in the group want to lose their 6 electrons. Group 17, the halogen group, are the elements in the second to last column. They have seven electrons in their outer shell, and they want to gain one electron. These are the most reactive nonmetals on the periodic table. Group 18, the noble gases, are nonreactive elements. This is because they have eight electrons in their outer shell, which means the shell is holding the maximum number of electrons. They neither want to gain or lose electrons, which is why they do not react with other elements. Finally, there is hydrogen. Hydrogen is in a group by itself. This is because it has only one electron in the outer shell. Since it only has one electron, it acts similar to the alkali metals as far as reacting with other elements, however, since it is a nonmetal it cannot be included in the alkali metal group. Color code the periodic table below based upon the categories given in the paragraphs above. (you should end up using 7 different colors. Also create a key to go along with the colors. Periodic Trends: One of the greatest aspects of the periodic table is that you can predict an element’s characteristics and behaviors based upon where it is on the table. Nucleus size: The size of the nucleus increases as you move from the top to the bottom of the table Number of protons: When you move from left to right across the table, the number of protons increases. Atomic mass: Atomic mass increases both as you move from the top down also as you move from left to right. Element reactivity: The trend for reactivity with the metal elements is that they become more reactive as you go from the top down and moving from the right to the left. It is the exact opposite for the nonmetals. Nonmetals increase in reactivity from the bottom up and from left to right. The one main point though is that group 18, the noble gasses are the exception. Even though they are the furthest to the right, they are nonreactive. Use the image below to answer the following questions. For the two metals, barium and silver (highlighted in blue) which one has: a. Larger nucleus b. More protons c. Higher atomic mass d. Higher reactivity For the two nonmetals, phosphorus and fluorine (highlighted in orange) which one has: a. Larger nucleus b. More protons c. Higher atomic mass d. Higher reactivity