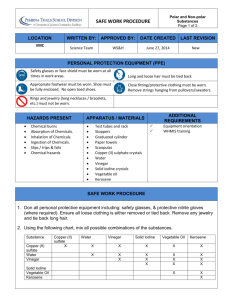
Name: Pd. Date: CHEMICAL PROPERTIES LAB Objective: The student will define and identify chemical properties and use them to identify and describe specific matter. Background: Physical properties are not the only way to describe matter. Matter also has chemical properties and both physical and chemical properties are needed to fully describe and identify specific matter. Materials: Substances A, B, C, D Beaker with water Beaker with vinegar (acetic acid) Bottle of iodine 3 eyedroppers 4 popsicle sticks 1 spot plate stirring rod Procedure: 1. 2. 3. 4. 5. 6. 7. Place a small amount of substance A into the bottom of one of the spots on your spot plate. Record the physical properties of substance in your data table. Add 3-5 drops of water to substance A in the plate. Stir gently with stirring rod. Rinse off stirring rod. Record your observations in the data table. Repeat the procedure for each of the four substances with each of the 3 liquids. Rinse and dry your spot plate. Substance Physical properties Properties after addition of water Properties after addition of vinegar Properties after addition of Iodine Identity of Substance A B C D Analysis Questions: 1. Were you able to distinguish the substances based on their physical properties alone? Explain your answer. 2. Write the definition of a Chemical Property: 3. Using the following information identify each of your substances and write them in your data table: a. Baking powder reacts with both vinegar and water b. Baking soda reacts with vinegar. c. Cornstarch reacts with iodine. d. Sugar does not react with water, vinegar or iodine. 4. Using your textbook list other chemical properties of matter. 5. Compare and contrast physica and chemical properties using a Venn Diagram.