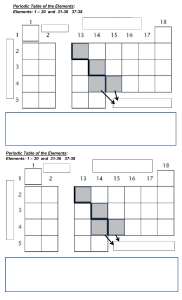
The Periodic Table The Ultimate Cheat-Sheet Elementary My Dear Watson…. Elements are distinguished by the number of protons Each element has unique properties How are they arranged? Is there a pattern? The “Original” Why Do We Need a Periodic Table? By 1700, only 13 elements were known The rate of discovery increased in the 18th century (Davy, Lavoisier, Priestly) But how could scientists know an element was “new?” Chemists needed a way to organize the elements How Was It Developed? In 1829, Dobereiner published a classification system using triads Triads are groups of 3 elements with similar properties But, not all elements could be grouped into triads Lothar Meyer In 1864, Lothar Meyer published an early version of the periodic table It contained 28 elements classified into 6 families by their valence (combining power) This was the first time that elements had been grouped and ordered according to their valence. Work on organizing the elements by atomic weight had hitherto been stymied by inaccurate measurements of the atomic weights. 7 de Chancourtois One of the first to notice periodicity In 1862 developed a cylindrical design Ignored due to “geological terms” How Was It Developed…. In 1865, Newlands classified elements into 11 groups Noticed that many groups differed by He called this his “Law of Octaves” “8” Development Other systems were explored… In 1869, Dmitri Mendeleev proposed his periodic table He played “chemical solitaire” on the train There were 60 elements to organize 1834-1907 How Did Mendeleev Do It? He organized the elements by increasing atomic mass and “combining power” He left a space in his table if an element was unknown In time, those spaces were filled in with elements that matched his predictions Elements’ Properties are Predicted Property Mendeleev’s Predictions in 1871 Observed Properties Scandium (Discovered in 1877) Molar Mass Oxide formula Density of oxide Solubility of oxide 44 g M2O3 3.5 g / ml Dissolves in acids 43.7 g Sc2O3 3.86 g / ml Dissolves in acids Gallium (Discovered in 1875) Molar mass Density of metal Melting temperature Oxide formula Solubility of oxide 68 g 6.0 g / ml Low M2O3 Dissolves in ammonia solution 69.4 g 5.96 g / ml 30 0C Ga2O3 Dissolves in ammonia Germanium (Discovered in 1886) Molar mass Density of metal Color of metal Melting temperature Oxide formula Density of oxide Chloride formula Density of chloride Boiling temperature of chloride 72 g 5.5 g / ml Dark gray High MO2 4.7 g / ml MCl4 1.9 g / ml Below 100 oC O’Connor Davis, MacNab, McClellan, CHEMISTRY Experiments and Principles 1982, page 119, 71.9 g 5.47 g / ml Grayish, white 900 0C GeO2 4.70 g / ml GeCl4 1.89 g / ml 86 0C Mendeleev Some people consider Meyer and Mendeleev the co-creators of the periodic table Most agree that Mendeleev's accurate prediction of the qualities of what he called eka-silicon (germanium), eka-aluminium (gallium) and eka-boron (scandium) qualifies him for deserving the majority of the credit for studies 14 Mendeleev did not know the structure of atoms and that the number of protons was unique for each element Now the periodic table is arranged by increasing atomic number The Modern Periodic Table Is an organized display of elements Is arranged so that elements with similar properties fall into the same group Is used to predict the behavior of elements The “Noble Gases” don’t easily react with other elements. Rows of the Periodic Table Rows of the PT are called “periods.” All of the elements in a period have the same number of energy levels for their valence (s & p) electrons Periods (cont.) Elements close to each other in the same period are more similar than those further away. K and Ca are similar K and Kr are very different So… Properties of the elements within a period change as we move across a period from left to right The pattern of properties within a period repeats as we move from one period to the next The Periodic Law When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties Regions of the PT 1) Metals The largest region of the PT ~ 80% Properties of Metals Excellent conductors of heat and electricity Usually lustrous, ductile, and malleable. Sodium metal Copper wire Gold charm 2) Nonmetals The second largest region of the PT Properties of Nonmetals Poor conductors of heat and electricity Most are gases or brittle solids at room temperature. Graphite Diamond Chlorine gas 3) Metalloids Have some properties of metals and some of nonmetals Silicon is useful in computers because they conduct electricity “moderately” Semi-conductors Other Uses of Metalloids Lasers Infrared sensors Alloys Glass products Added Impurities The Elements at the Bottom These are the lanthanides and actinides Glenn Seaborg “moved” these Special Groups of Elements Group 1A – the Alkali Metals The name alkali comes from the Arabic al aqali, meaning “the ashes.” Wood ashes are rich in compounds containing sodium and potassium Group 2A – the Alkaline Earth Metals Group 7A – the Halogens Literally means “salt former” 30 The Representative Elements These are the elements in Groups 1A – 7A They represent a wide range of properties Metals, nonmetals, and metalloids Solids, liquid (Br), and gases The highest level s & p orbitals are NOT filled 31 Examples Electron configurations for Group 1A Electron configurations for Group 4A 32 Group Number and e- Number For any representative element, its group number equals the number of electrons in the highest occupied energy level (valence electrons). Group 1A Group 4A 33 Noble Gases NOT considered representative elements Unreactive Uses: Krypton is mixed with Argon in fluorescent lights (also to render Superman inert) Neon is used for signs Helium is used in weather and toy balloons What About The “Ones in the Middle” These have different letter designations, depending on the table These are also called Transition metals Inner transition metals 35 Transition Metals The highest occupied s sublevel and a nearby d sublevel contain electrons These elements are also called d -block elements 36 The Inner Transition Metals AKA: the lanthanides and actinides. The highest occupied s sublevel and a nearby f sublevel generally contain electrons. 37 f-orbitals 38 Blocks of Elements 39 Rare Earth Elements Scandium, Yttrium, Lanthanum, and Cerium through Lutetium Used in electronics Thulium (Tm) – lasers & x-rays Neodymium (Nd) - magnets Not rare – hard to separate Elements very similar Charges are 3+ 40 The Racetrack Design