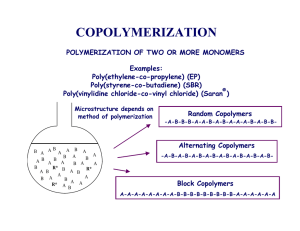
International Journal of Trend in Scientific Research and Development (IJTSRD) UGC Approved International Open Access Journal ISSN No: 2456 - 6470 | www.ijtsrd.com | Volume - 1 | Issue – 5 Significance of Amphiphilic Block Copolymer Micelles and its Characteristics Tejas Joshi Department of Chemistry, UGC NON-SAP & DST-FIST Sponsored Department, Maharaja Krishnakumarsinhji Bhavnagar University, Bhavnagar, India ABSTRACT Amphiphilic block copolymers (ABCs) have been used broadly in pharmaceutical applications. One of the most widely used drug delivery systems is the self assembly of ABCs carriers in micelle forms in aqueous environment. Block copolymers have low toxicity and due to their nontoxic properties and surface-activity they have found application in the areas of biomaterials, protein separation, drug delivery and cardiovascular therapeutics and as industrially important surfactants. ABCs micelles have been the focus of research for the last many decades. Research in the field has been increasingly focused on achieving enhanced stability of the micellar assembly, prolonged circulation times and controlled release of the drug for optimal targeting. Keywords: Block copolymer, amphiphilic, micelles, surfactants I. INTRODUCTION Simple polymer can be formed by joining monomer units and become long chain polymer which is revealed by Figure 1. Here monomer molecules united to become long chain which is known as polymer. Figure 1 Formation of polymer Block copolymer is a linear arrangement where two often-incompatible blocks obtained from different monomers are covalently linked together. This basic structure architecture in block copolymers provided two distinct moieties behaving differently in solution and thus these polymers possess structural resembles to surface-active agents. Figure 2 is generalized structure of block copolymer. `@ IJTSRD | Available Online @ www.ijtsrd.com | Volume – 1 | Issue – 5 | July-Aug 2017 Page: 407 International Journal of Trend in Scientific Research and Development (IJTSRD) ISSN: 2456-6470 Figure 2 Structure of block copolymer There are various possibilities to form different structure of block copolymers. In this way diblock (AB), triblock (A-B-A and B-A-B) and multiblock (or segmented) polymers are possible. Amphiphilic drugs bear an ionic or nonionic polar headgroup and a hydrophobic portion. They tend to self-associate as micelles in aqueous solution in a surfactant like manner. Block copolymers possess unique structural features resembling to that of surfactants thus they do adsorb onto interfaces and self-assemble to form micelles in solutions. Figure 3 Types of homopolymer and copolymer A number of triblock PEO-PPO-PEO copolymers have been commercially available since 1950’s under the generic name Poloxamers and the trademarks Pluronic® (BASF, Mount Olive, NJ, USA) and Synperonic® (ICI, Cleveland, UK) [1-5]. The possibilities of large variation in total molecular weight and block composition have led to a large number of products. The molecular weights of the commercially available copolymers are typically in the 2000-20000 Da range and their PEO contents in the 10-80% ranges. II. TYPES OF COPOLYMERS Polymer may be homopolymer or copolymer depending upon the type of monomer unit attached to form long chain of polymer. Copolymers classify according to shape and building architecture as well as arrangement of monomeric group in polymer chain. Basically copolymer can be classify either by alternating, random, graft and block copolymer (Figure 3). Further block copolymer may sub classify as di-block or tri-block or multi-block copolymer (Figure 4). Figure 4 Types of block copolymer The triblock architecture is the result of the polymerization process where first a PPO homopolymer is synthesized (constituting the middle block of the copolymer) by the addition of propylene oxide to the hydroxyl groups of propylene oxide to the hydroxyl groups of propylene glycol, and then ethylene oxide is added at both ends of the PPO block to form the PEO endblocks. Figure 5 represents the formation of micelles from different polymers. `@ IJTSRD | Available Online @ www.ijtsrd.com | Volume – 1 | Issue – 5 | July-Aug 2017 Page: 408 International Journal of Trend in Scientific Research and Development (IJTSRD) ISSN: 2456-6470 Figure 5 represents the formation of micelles from different polymers III. STIMULI RESPONSIVE COPOLYMERS BLOCK Stimuli Responsive polymers have some environmental responding properties so that they can swell or shrink corresponding to a small variation of temperature, pH, and ionic strength. Their potential applications in drug delivery, separation, and bioswitch have been widely suggested. [7 - 9] Poly (N-isopropylacrylamide) (PNIPAM) is a typical example of such “smart” polymers, which can undergo the coil-to-globule transition in water at its low critical solution temperature (LCST ~32 °C) [10 – 12]. Micelles and aggregates from PS–PEO diblock copolymers have been investigated by a number of techniques for their potential use for encapsulation of hydrophobic materials in an aqueous suspension. Various morphologies, spheres, rods, lamellae, and vesicles, in dilute aqueous solutions depending on the molecular characteristics of PS–PEO diblock copolymers have been reported. [13 – 16]. IV. Figure 6 Self –assembly of amphiphilic block copolymers A Pluronic grid (Figure 7) was developed to provide graphic representation of the relationship between copolymer structure, physical form, and surfactant characteristics [17]. SOLUTION PROPERTIES OF AMPHIPHILIC BLOCK COPOLYMER SURFACTANTS SYSTEMS Formation of polymeric micelles from singlr block copolymer is shown in below Figure 6. `@ IJTSRD | Available Online @ www.ijtsrd.com | Volume – 1 | Issue – 5 | July-Aug 2017 Page: 409 International Journal of Trend in Scientific Research and Development (IJTSRD) ISSN: 2456-6470 the surfactants performance in different industrial formulations, Thus there has been a growing interest both in basic and applied research. Likewise, interaction of Pluronic® with sodium dodecyl sulphate (SDS) using several techniques has also been studied [35 – 38]. V. Figure 7 Pluronic Grid Temperature plays an important role on the aggregation behavior. The presence of micellar aggregates in solution gives rise to a certain value of intermicellar potential. At certain value of temperature, this potential reaches a very high value. At initial temperature, the micellar aggregates are hydrated by the water molecules, which is responsible for screening the van der Waals forces between the species. The increase in the temperature results in dehydration of the copolymers blocks. As a result, the van der Waals force (attractive potential between the species) increases. At certain value of temperature, this attractive force reaches a very high value to impart phase separation. The performance based properties of these polymeric surfactants can be enhanced in presence of salts, hydrotropes and organic additives such as alcohols, amines etc. Recent studies on aqueous solution behaviour of PEO–PPO– PEO block copolymers involve micellization and phase behaviour and several techniques have been employed to investigate the self-assemblies formed. These include static and dynamic light scattering [18-19], SANS [20 – 21], static and time resolved fluorescence [22-24], FTIR [25- 26], microcalorimetry [27], cryo- TEM [28], surface tension [29 - 30], viscosity [18, 31], oscillatory shear measurements [18, 32], and sound velocity/ultrasonic absorption method [33 - 34] etc. The aggregation and aggregate structures have been examined at different temperatures and in the presence of additives like inorganic salts, nonelectrolytes, hydrotropes and ionic surfactants. Micellar solutions of mixed surfactants exhibit remarkably different behavior from those of single surfactants and this property can be used to optimize APPLICATIONS The wide variety of application of polymer-surfactant mixed systems in aqueous solutions has thus motivated both chemists and biologists. Block copolymers have low toxicity and due to their nontoxic properties and surface-activity they have found application in the areas of biomaterials, protein separation, drug delivery and cardiovascular therapeutics and as industrially important surfactants. Amphiphilic poly(ethylene oxide)–poly(propylene oxide) block copolymers are thermoresponsive materials that display unique aggregation properties in aqueous medium. In solutions at concentration above a critical concentration (CMC) surfactant molecules tend to aggregate and form micelles. In the presence of large polymers, surfactant micelles can form selfassembled complexes. These complexes play a fundamental role in a broad range of industrial applications, including colloid stabilization and detergency, and are increasingly found in commercial surfactant formulations such as foods, pharmaceuticals, cosmetics, textiles, polymers, paints, and paper [39- 42]. Over the years, therefore, micelles have been of interest to pharmacological scientists either as drug delivery systems or as targeting systems. Amphiphilic drugs solubilize in body fluids and interact with membranes in the organism before they reach their final targets. Aggregates of these amphiphilic drugs could act as their own carriers. Future Challenges needs suitable, effective drug delivery or targeting systems. VI. CONCLUSIONS Over the past two decades, new surfactant molecules have been appearing at a relatively rapid pace. Scientific curiosity has also driven surfactant science research to focus on surfactant molecules having interesting fabricated shapes and structures. Fundamental knowledge of surfactant is very essential although surfactant science is now very well established discipline and there is still room for new molecules designed for specific purposes and new applications. `@ IJTSRD | Available Online @ www.ijtsrd.com | Volume – 1 | Issue – 5 | July-Aug 2017 Page: 410 International Journal of Trend in Scientific Research and Development (IJTSRD) ISSN: 2456-6470 ACKNOWLEDGEMENT Dr. Tejas P Joshi would like to thank Prof. N. C. Desai, Head, Dept. of Chemistry, Maharaja Krishnakumarsinhji Bhavnagar University, Bhavnagar, for honest support. REFERENCES 1. J. Jacobs, R. A. Anderson, and T. R.Warson, J. Pharm. Pharmacol., 24 (1972) 586. 2. J. M. G. Cowie, and Sirianni, J. Am. Oil Chem. Soc., 43 (1966) 572. 3. J. M. Corkill, J. F. Goodman, and T. Walker, Trans. Faraday Soc., 61 (1965) 589. 4. I. R. Schmolka, Polyalkylene oxide block copolymers, in Nonionic surfactants, Surf. Sci. Ser., Marcel Dekker, New York, 1 (1967) 300. 5. H. E. Garret, Surface Active Chemicals, Pergamon, New York, 1975. 6. S. Kumar, V. K. Aswal, A. Z. Naqvi, P. S.Goyal, Kabir-ud-Din, Langmuir, 17 (2001) 2549. 7. F. Bignotti, M. Penco, L. Sartore, I. Peroni, R. Mendichi, M. Casolaro, A.D. Amore, Polymer 41 (2000) 8247. 8. T. V.Burova, N.V. Ginberg, V. Y. Grinberg, E.V. Kalinina, V.I. Lozinsky, V.O. Aseyev, S. Holappa, H. Tenhu, A.R. Khokhlov, Macromolecules 38 (2005) 1292. 9. T. Okano, A. Kikuchi, Y. Sakurai, Y. Takei, N.J. Ogata, Controlled Release 36 (1995) 125. 10. K. Otake, H. Inomata, M. Konno, S. Saito, Macromolecules 23 (1990) 283. 11. K. Kubota, K. Hamano, N. Kuwahara, S. Fujishige, I. Ando, Polymer 22 (1990) 1051. 12. K. Kubota, S. Fujishige, I. Ando, J. Phys. Chem. 94 (1990) 5154. 13. C. Zhao, M.A. Winnik, G. Riess, M.D. Croucher, Macromolecules 6 (1990) 514. 14. R. Xu, M.A.Winnik, F.R. Hallett, G. Riess, M.D. Croucher, Macromolecules 24 (1990) 87. 15. F. Calderara, Z. Hruska, G. Hurtrez, J. P. Lerch, T. Nugay, G. Riess, Macromolecules 27 (1994) 1210. 16. K. Yu, A. Eisenberg, Macromolecules 29 (1996) 6359. 17. Pluronic and Tetronic Surfactants, Technical Brochure, BASF Corp., Parsippany, NJ (1989). 18. P. Bahadur, K. Pandya, Langmuir 8 (1992) 2666. 19. J. Mata, D. Varade, G.Ghosh, P.Bahadur , Colloids Surf. A: Physicochem. Eng. Aspects 245 (2004) 69. 20. K. Mortensen, Colloids Surf. A: Physicochem. Eng. Asp. 183 (2001) 277. 21. V.K. Aswal, P. S. Goyal, J. Kohlbrecher, P. Bahadur, Chem. Phys. Lett. 349 (2001) 458. 22. K. Nakashima, K. Takeuchi Appl. Spectrosc. 55 (2001) 237. 23. M.S. Bakshi, G. Kaur, I. Ahmad, Colloids Surf A: Physicochem.Eng. Asp. 253 (2005) 1. 24. T. Nivaggioli, B. Tsao, P. Alexandridis, T.A.Hatton, Langmuir 11 (1995) 119. 25. Y. Su, J. Wang, H. Liu, Macromolecules 35 (2002) 6426. 26. Y. Su, X. Wei, H. Liu, J. Colloid Inter. Sci. 264 (2003) 526. 27. S.K. Nixon, S. Hvidt, C. Booth, J Colloid Inter. Sci 280 (2004) 219. 28. K. Mortensen, Y. Talmon, Macromolecules 28 (1995) 8829. 29. J. Mata, T. Joshi, D. Varade, G. Ghosh, P. Bahadur, Colloids Surf. A: Physicochem. Eng. Asp. 247 (2004) 1. 30. J. Dong, B.Z. Chowdhary, S.A. Leharne, Colloids Surf. A: Physicochem. Eng. Asp. 212 (2003) 9. 31. R. Ganguly, V. K. Aswal, P.A. Hassan, I. K. Gopalakrishnan, J.V. Yakhmi, J. Phys. Chem. B 109 (2005) 5653. 32. S. Hvidt, Colloids Surf A: Physicochem. Eng. Asp. 112 (1996) 201. 33. P. Taboada, S. Barbosa, V. Mosquera Langmuir 20 (2004) 8903. 34. G. Waton, B. Michelles, R. Zana, J. Colloid Inter. Sci. 212 (1999) 593. 35. M. J. Kositza, G.D. Rees, A. Holzwarth, J.F. Holzwarth Langmuir 16 (2000) 9035. 36. Y. Li, R. Xu, S. Couderc, D.M. Bloor, E. WynJones, J.F. Holzwarth, Langmuir 17 (2001) 183. 37. S. Dai, K.C. Tam, L. Li, Macromolecules 34 (2001) 7049. 38. A. J. Konop, R. H. Colby, Langmuir, 15 (1999) 58. 39. Y. Li, R. Xu, D.M. Bloor, J.F. Holzwarth, E. Wyn-Jones Langmuir. 16 (2000) 10515. [91] 40. L. E. Bromberg, M Temchenko, R. H. Colby, Langmuir, 16 (2000) 2609. 41. R. H. Colby, N. Plucktaveesak, L. E. Bromberg, Langmuir, 17 (2001) 2937. 42. N. Plucktaveesak, A.J. Konop, Colby R.H., J. Phys. Chem B 107 (2003) 8166. `@ IJTSRD | Available Online @ www.ijtsrd.com | Volume – 1 | Issue – 5 | July-Aug 2017 Page: 411