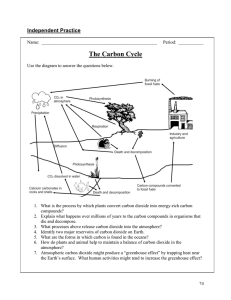
SPECIES, COMMUNITIES AND ECOSYSTEMS Species 🐻🐻🐻 A species is a group of organisms that can potentially interbreed to produce fertile and viable offspring. ● Members of a single species are unable to produce fertile and viable offspring with members from a different species ● When 2 different species do produce offspring by crossbreeding, these offspring are called hybrids and they are reproductively sterile (e.g. liger, mule) 🐻❌🦑 🐻🐻🐻 A population is a group of organisms of the same species that are living in the same area at the same time ● Organisms that live in different regions, different populations, are reproductively isolated and unlikely to interbreed, however are classified as the same species if interbreeding is functionally possible Ecology Terms 🐻🐻🐻 Species - a group of organisms that can potentially interbreed to produce fertile and viable offspring. Population - a group of organisms of the same species, living in the same area at the same time. Community - a group of populations living together and interacting with each other within a given area. Habitat - the environment in which a species normally lives, or the location of a living organism. Ecosystem - a community and its abiotic environment (i.e habitat) Ecology - the study of the relationship between living organisms, or the relationship between living organisms and their environment. 🐻🐻🐻 🐻🐰🥕 ⛰🏝 🐻🐰🥕➕🌲🌲🌦 Modes of Nutrition Living organisms obtain chemical energy in one of 2 ways. Some species have either an autotrophic or heterotrophic method of nutrition, but some may have both! 🌳🌻 Autotrophs ● Autotrophs synthesise their own organic molecules from simple inorganic substances e.g. carbon dioxide ● Energy for this process is derived from sunlight (photosynthesis) or via the oxidation of inorganic molecules (chemosynthesis) ● Because autotrophs synthesise their own organic molecules they are commonly referred to as producers 🐯🐻🐰 Heterotrophs ● Heterotrophs obtain organic molecules from other organisms, either living, recently killed, or their nonliving remains and detritus (organic waste of dead organisms) ● Because heterotrophs cannot produce their own organic molecules and obtain it from other sources, they are called consumers Mixotrophs ● Certain unicellular organisms may on occasion use both forms of nutrition, depending on resource availability ● Euglena gracilis possess chlorophyll for photosynthesis but may also feed on detritus Further classification of species based on their mode of nutrition ● Autotrophs produce their own organic molecules using either light energy or energy derived from the oxidation of chemicals ○ Photoautotrophs use photosynthesis to make organic compounds using energy derived from the sun ○ Chemoautotrophs use chemosynthesis to make organic compounds using energy derived from the oxidation of chemicals ● Heterotrophs obtain organic molecules from other organisms via one of three methods ○ Consumers ingest organic molecules from living or recently killed organisms ○ Detritivores ingest organic molecules found in the non-living remnants of organisms (detritus, humus) ○ Saprotrophs release digestive enzymes and then absorb the external products of digestion (decomposers) Autotrophs Autotrophs synthesise organic molecules from simple inorganic substances ● Most autotrophs derive the energy for this process from sunlight via photosynthesis ☀ ● Some may derive the needed energy from the oxidation of inorganic molecules via chemosynthesis 🧪 🌨🌲 Autotrophs obtain the simple inorganic substances required for this process from the abiotic environment (air, water, soil… non living) ● These nutrients include carbon, nitrogen, hydrogen, oxygen and phosphorus, and they are obtained from the air, water or soil Heterotrophs may also obtain some simple inorganic substances from the environment, but principally obtain their carbon and nitrogen from the organic molecules produced by autotrophs. Heterotrophs Heterotrophs obtain organic molecules from other organisms via different feeding mechanisms and different food sources. Consequently, heterotrophs can be differentially classified according to their feeding pattern. 🥩🥦 Consumers ● Consumers are heterotrophs that feed on living organisms by ingestion ● Herbivores are consumers that feed on plant matter (cows, sheep, rabbit) ● Carnivores are consumers that feed on animal matter (tiger, beary) ● Omnivores are consumers that have a diet composed of both plant and animal matter (humans, pandas) 🐼👩🏻🍳 🐮🐑🐰 🦁🐯🐻 💀👻🥩🦅 Scavengers (pretend this is a vulture) ● Scavengers are a type of consumer that feed on dead and decaying carcasses rather than hunting live prey ● E.g. hyenas, carrion birds such as crows, vultures 💀👻💩🦀🐌 Detritivores ● Detritivores are a type of heterotroph that obtains nutrients from non-living organic sources, such a as detritus (waste or debris) and humus (organic components of soil, formed by the decomposition of leaves and other plant material by soil microorganisms) ● Detritus is dead, particulate organic matter, such as decaying organic material and fecal matter POO ● Humus is the term given to the decaying leaf litter intermixed within the topsoil ● Detritivores include, earthworms, snails, crabs, dung beetles 🍄🦠🧪 Saprotrophs ● Saprotrophs are heterotrophs that obtain organic nutrients from dead organisms by external digestion, so they don’t ingest it! ● They live on, or in, non-living organic matter ● They secrete digestive enzymes into it and absorbs the products of digestion ● Unlike other types of heterotrophs, saprotrophs do not ingest food, but use enzymatic secretion to facilitate external digestion ● Because saprotrophs facilitate the breakdown of dead organic material, they are commonly referred to as decomposers ● Examples of saprotrophs include bacteria and fungi, mushroom, mold ● Bonus notes: So you know how viruses make u sick right? Well bacteria make u sick differently, they digest things right so they release a lot of chemicals, which may damage tissues in your body, those chemicals might be toxic. Nutrient Cycling Nutrients are the materials required by an organism. It includes elements such as carbon, nitrogen and phosphorus. The supply of inorganic nutrients on Earth is finite, new elements cannot simply be created and so are in limited supply. Chemical elements are constantly recycled after they are used, since there is a limited supply ● Autotrophs obtain inorganic nutrients from the air, water and soil and convert them into organic compounds ● Heterotrophs ingest these organic compounds and use them for growth and respiration, releasing inorganic byproducts ● When organisms die, saprotrophs decompose the remains and free inorganic materials into the soil ● The return of organic nutrients to the soil ensures the continual supply of raw materials for the autotrophs Mesocosms 🌲🌲🌦 🐻🐰🥕) and Ecosystems describe the interaction between biotic components (community abiotic components (habitat ) ● Ecosystems are largely self contained and have the capacity to be self sustaining over long periods of time 3 components are required for sustainability in an ecosystem: 1. Energy availability - light from the sun provides the initial energy source for almost all communities 2. Nutrient availability - saprotrophic decomposers ensure the constant recycling of inorganic nutrients within an environment 3. Recycling of wastes - certain bacteria can detoxify harmful waste byproducts, for example, denitrifying bacteria such as N itrosomonas Mesocosms are enclosed environments that allow a small part of a natural environment to be observed under controlled conditions. A terrarium is a small transparent container, either plastic or glass, in which selected plants or animals are kept and observed. You need to know how to make a self-sustaining terrarium! You can make one using a glass or plastic bottle with a lid. 1. Building a verdant foundation a. Add a bottom layer of pebbles, gravel or sand - this layer exists for drainage. The smaller the vessel the thinner rock layers required. b. Add a second thin layer of activated charcoal, this will prevent mold and help to aerate the soil c. Spread a thin cover of sphagnum moss, or organic coffee filter, to create a barrier between the lower layers and soil d. The final layer is the pre-moistened growing medium, such as soil potting mix 2. Select the right plants a. Choose plants that are slow growing and thrive in a bit of humidity, such as ferns, club moss b. Inspect the plant thoroughly for any signs of disease or insects before introducing to the terrarium 3. Maintaining appropriate conditions a. Ensure the terrarium is placed in a location that provides a continuous source of light b. Locate the terrarium in a place that does not experience fluctuating temperature conditions (avoid direct sunlight!) c. Do not initially over water the plants, once the right humidity is established, a terrarium can go months without watering! d. Occasional pruning (trim plants) may be required, but then, as level of soil nutrients decrease, plant growth should slow down 😳) Chi-Squared Test (again? The presence of two species within a given environment will be dependent upon potential interactions between them. If two species are typically found within the same habitat, they show a positive association. ● Species that show a positive association include those that exhibit predator-prey or symbiotic relationships If two species tend not to occur within the same habitat (react less), they show a negative association ● Species will typically show a negative association if there is competition for the same resources ● One species may utilise the resources more efficiently, precluding survival of the other species (competitive exclusion) ● Both species may alter their use of the environment to avoid direct competition (resource partitioning) If two species do not interact, there will be no association between them and their distribution will be independent of one another. Quadrat Sampling The presence of two species within a given environment can be determined using quadrat sampling. A quadrant is a rectangular frame of known dimensions that can be used to establish population densities. ● Quadrats are placed inside a defined area in either a random arrangement or according to a design ● The number of individuals of a given species is either counted or estimated via percentage coverage ● The sampling process is repeated many times in order to gain a representative data set Quadrat sampling is not an effective method for counting motile organisms, it is used for counting plants and sessile animals ● In each quadrat, the presence or absence of each species is identified ● This allows for the number of quadrats where both species were present to be compared against the total number of quadrats Chi-Squared Test A chi-squared test can be applied to data generated from quadrat sampling to determine if there is a statistically significant association between the distribution of two species. The presence or absence of two species of scallop was recorded in fifty quadrats (1m2 sized quadrat) on a rocky seashore. The following distribution pattern was observed: ● 6 quadrats had both species ● 15 quadrats had king scallop only ● 20 quadrats had queen scallop only ● 9 quadrats had neither species QUEEN SCALLOP KING SCALLOP Present Absent Total Present 6 15 21 Absent 20 9 29 Total 26 24 50 Step 1 - Set up a hypothesis ● Null hypothesis (H0): there is no significance difference between the distribution of two species (distribution is random) ● Alternative hypothesis (H1): there is a significant difference between the distribution of species (the species are associated) Step 2 - Construct a table of frequencies Times the totals (we are finding the expected values! E) QUEEN SCALLOP KING SCALLOP Present Absent Total Present 26 x 21 / 50 = 10.9 24 x 21 / 50 = 10.1 21 Absent 26 x 29 / 50 = 15.1 24 x 29 / 50 = 13.9 29 Total 26 24 50 Apply the Chi-squared formula Where ∑ = Sum, O = Observed frequency, E = Expected frequency QUEEN SCALLOP KING SCALLOP PRESENT PRESENT ABSENT OBSERVED 6 15 EXPECTED 10.9 10.1 ABSENT (O−E)2 E 2.20 2.38 OBSERVED 20 9 EXPECTED 15.1 13.9 (O−E)2 E 1.59 1.73 X2 = 2.20 + 2.38 + 1.59 + 1.73 = 7.90 Determine the degree of freedom 2 species - 1 = 1 Degree of freedom is 1 Look at table of critical values (given) Always look at 0.05 (5%) ● If the X2 value is equal or greater than the critical value, reject the null hypothesis H0 ● Ok the critical value is 3.841. ● 7.90 > 3.841, hence we reject H0 and accept H1 which states that there is a significant difference between observed and expected frequencies ● So it means that these two species do not tend to be present in the same area, so we infer that there is a negative association between them Energy Flow Energy Source All green plants, and some bacteria, are photoautotrophic☀, meaning they use sunlight as a source of energy. ● As a result, light is the initial source of energy for almost all communities ● In a few ecosystems the producers are chemoautotrophic bacteria, which use energy derived from chemical processes Light energy is absorbed by photoautotrophs and is converted into chemical energy via photosynthesis. ● This light energy is used to make organic compounds (e.g sugars) from inorganic sources (e.g CO2) ● Heterotrophs ingest these organic compounds in order to derive their chemical energy (ATP) ● When organic compounds are broken down via cell respiration, ATP is produced to fuel metabolic processes Energy Flow Energy enters most ecosystems as sunlight, where it is converted into chemical energy by producers via photosynthesis. This chemical energy is stored in carbon compounds (organic molecules) and is transferred to heterotrophs via feeding. Trophic Levels The position an organism occupies within a feeding sequence is known as a trophic level. ● Producers always occupy the 1st trophic level in a feeding sequence ● Primary consumers feed on producers, so they occupy the 2nd trophic level ● Secondary consumers feed on primary consumers occupy the 3rd trophic level ● Tertiary consumers feed on secondary consumers so they occupy the 4th trophic level Food Chains A food chain shows the linear feeding relationships between species in a community ● Arrows represent the transfer of energy and matter as one organism is eaten by another (arrows point in the direction of energy flow) ● The first organism in a food chain is always a producer, followed by the consumers (primary, secondary, tertiary) ● → → → 🥦🐰🦊🐻 Energy Loss Energy stored in organic molecules, such as sugars and lipids, can be released by cell respiration to produce ATP. ● This ATP is then used to fuel metabolic reactions required for growth and homeostasis ● A by-product of these chemical reactions is heat (thermal energy), which is released from the organism Not all energy stored in organic molecules is transferred via heterotrophic feeding, some of the chemical energy is lost by: ● Being excreted as part of the organism’s faces ● Remaining unconsumed as the uneaten portions of the food Living organisms cannot convert heat to other forms of energy, and Heat is lost from ecosystems The chemical energy produced by an organism can be converted into a number of forms, including: ● Kinetic energy during muscular contractions ● Electrical energy during the transmission of nerve impulses ● Light energy when producing bioluminescence (lantern fish) All of these reactions are exothermic and release thermal energy (heat) as a by-product ● Living organisms cannot turn this heat into other forms of usable energy ● This heat energy is released from the organism and is lost from the ecosystem, unlike nutrients, which are recycled ● Hence, ecosystems require a continuous influx of energy from an external source, such as the sun Energy Efficiency When energy transformations take place in living organisms the process is never 100% efficient ● Most of the energy is lost to the organism, either used in respiration, released as heat, excreted in faeces, or unconsumed ● Typically, energy transformations are about ~10% efficient, with about 90% of available energy lost between trophic levels ● The amount of energy transferred depends on how efficiently organisms can capture and use energy (usually between 5-20%) As energy is lost between trophic levels, higher trophic levels store less energy as carbon compounds, and so higher trophic levels have less biomass ● Biomass is the total mass of a group of organisms, consisting of the carbon compounds contained in the cells and tissues ● Because carbon compounds store energy, scientists can measure the amount of energy added to organisms as biomass ● Biomass diminishes along food chains with the loss of carbon dioxide, water and waste products (e.g urea) to the environment Because energy and biomass is lost between each level of a food chain, the number of potential trophic levels are limited. ● Higher trophic levels receive less energy/biomass from feeding and so need to eat larger quantities to obtain sufficient amounts ● Because higher trophic levels need to eat more, they expend more energy and biomass hunting for food ● If the energy required to hunt food exceeds the energy available from the food eaten, the trophic level becomes unviable Pyramids of Energy A pyramid of energy is a graphical representation of the amount of energy at each trophic level of a food chain. ● They are expressed in units of energy per area per time (kJ m–2 year–1) Pyramids of energy will never appear inverted as some of the energy stored in one source is always lost upon transfer ● Each level should be roughly one tenth of the size of the preceding level, as energy transformations are ~10% efficient ● The bottom level will always represent the producers, with subsequent levels representing consumers (primary, secondary, etc) Carbon Cycling The Carbon Cycle The carbon cycle is a biogeochemical cycle whereby carbon is exchanged between the different spheres of the Earth ● There are 4 spheres ○ Atmosphere (air) ○ Lithosphere (ground) ○ Hydrosphere (water/oceans) ○ Biosphere (living things ● These 4 spheres are also known as carbon sinks (reservoirs), so in the diagram you should label the sinks. Carbon is exchanged between a variety of forms, including ● Atmospheric gases, mainly carbon dioxide and also methane ● Oceanic carbonates, including bicarbonates dissolved in water, and calcium carbonate in corals and shells ● Organic materials, including the carbohydrates, lipids and proteins found in all living things ● Non living remains, such as detritus and fossil fuels Different processes facilitate the cycling of carbon between the different forms e.g. feeding, combustion. Drawing the carbon cycle Carbon Source 1: Carbon Compounds Carbon is found in the form of organic molecules in producers and consumers Autotrophs, such as all plants and algae, convert inorganic carbon dioxide into organic compounds via photosynthesis ● These organic compounds include the carbohydrates, lipids and proteins required by the organism for survival Since autotrophs use carbon dioxide for photosynthesis, the levels of carbon dioxide within the organism should always be low ● In other words, carbon dioxide should always be at a higher concentration in the atmosphere, or water ● This concentration gradient ensures that carbon dioxide will passively diffuse into the autotrophic organism as required ● In aquatic producers, carbon dioxide can usually diffuse directly into the autotroph, whereas in terrestrial plants, diffusion occurs at stomata Heterotrophs cannot synthesise their own organic molecules and instead obtain carbon compounds via feeding. Role of cell respiration All organisms may produce the chemical energy, ATP, required to power metabolic processes via the process of cell respiration. ● So, cell respiration produces ATP, which is used to power metabolic processes ● Cell respiration involves the breakdown of organic molecules (carbon compounds) and produces carbon dioxide as a by-product ● The buildup of carbon dioxide in respiring tissues creates a concentration gradient, allowing it to be removed by passive diffusion In autotrophs, the uptake of carbon dioxide by photosynthesis may at times be balanced by the production of carbon dioxide by respiration ● This is known as the compensation point, at which the net carbon dioxide assimilation is zero (intake = output) Similarly, the amount of carbon dioxide in the environment will be determined by the level of respiration and photosynthesis ● If there is more net photosynthesis than cell respiration occuring in the biosphere, atmospheric carbon dioxide levels should drop, and vice versa! Carbon Source 2: Calcium Carbonate (Aquatic Conversions) Carbon dioxide dissolves in water and some of it will remain as a dissolved gas, however the remainder will combine with water to form carbonic acid ● ● ● ● ● ● CO2 + H2O ⇄ H2CO3 (carbonic acid) Carbonic acid will then dissociate to form hydrogen carbonate ions H2CO3 ⇄ HCO3– + H+ (bicarbonate ion) This conversion also releases hydrogen ions (H+), which is why pH changes when carbon dioxide is dissolved in water, more acidic The bicarbonate ion also likes to dissociate into carbonate ions HCO3– ⇄ CO32- (carbonate ion) + H+ Aquatic autotrophs absorb both dissolved carbon dioxide and hydrogen carbonate ions and use them to produce organic compounds. When the carbonate ions come into contact with the rocks and sediments on the ocean floor, they acquire metal ions. This commonly results in the formation of calcium carbonate and the subsequent development of limestone. ● CO32- + Ca2+ ⇄ CaCO3 (calcium carbonate) Living animals may also combine the hydrogen carbonate ions with calcium to form calcium carbonate ● This calcium carbonate forms the hardered exoskeletons of coral, as well as forming the main component of mollusca shells ● When the organism dies and settles to the sea floor, these hard components may be fossilised in the limestone Carbon Source 3: Methane Methane is produced from organic matter in anaerobic conditions by methanogenic archaeans and some diffuses into the atmosphere or accumulates in the ground. Methanogens are archaea microorganisms (look like bacteria but not bacteria, another type) that produce methane (CH4) as a metabolic by-product in anaerobic conditions. Anaerobic (no oxygen) conditions where methanogens may be found include: ● Wetlands, e.g. swamps and marshes ● Marine sediments e.g. in the mud of lake beds ● Digestive tract of ruminant animals (funny shape stomach) e.g. cow, sheep, goat Methanogens produce methane from the by-products of anaerobic digestion, principally acetic acid and carbon dioxide ● So then when methanogens respire anaerobically, they produce acetic acid, carbon dioxide and hydrogen. These 3 products undergo some further change to produce methane. ● Acetic acid → Methane and Carbon Dioxide (CH3COO– + H+ → CH4 + CO2) ● Carbon Dioxide and Hydrogen → Methane and Water (CO2 + 4H2 → CH4 + 2H2O) Methane may either accumulate under the ground, or diffuse into the atmosphere. ● ● When organic matter is buried in anoxic conditions (no oxygen), such as seabeds, then deposits of methane as a form of natural gas may form underground ○ Decomposing bodies, some bacteria or methanogens ?? may release methane also Rising global numbers of domesticated cattle may be increasing the levels of methane being released into the atmosphere Oxidation of methane into carbon dioxide and water in the atmosphere When methane is released into the atmosphere as a result of anaerobic reactions, it only persists for ~12 years. Methane will be naturally oxidised to form carbon dioxide and water, in vapor form (CH4 + 2O2 → CO2 + 2H2O) ● This is why methane levels in the atmosphere are not very large, even though significant quantities are being produced. Carbon Source 4: Fossil Fuels Plants and animals may decompose via soil bacteria, and then they may be fossilised into fossils, and then extracted into fuels. Partial Decomposition In many soils, saprotrophic bacteria and fungi, and detritivores, will decompose dead organisms and return nutrients to the soil for cycling. ● This decomposition process requires oxygen, as cell respiration is required to fuel digestive reactions Water logged regions may lack oxygenated air spaces within the soil and thus possess anaerobic conditions ● Anaerobic respirations by organisms in these regions produces organic acids, such as acetate acid (methanogens), resulting in acidic conditions ● Saprotrophic bacteria and fungi cannot function effectively in anaerobic and acidic conditions, preventing decomposition And since this organic matter is not fully decomposed in waterlogged soils, carbon rich molecules remain in the soil and form peat ● When deposits of peat are compressed under sediments, the heat and pressure force out impurities and remove moisture ● The remaining material has high carbon concentration and undergoes a chemical transformation to produce coal ● Strange peat question: so apparently, acidic conditions, anaerobic conditions and the presence of organic matter favours the production of peat So coal only forms in marshy areas, waterlogged anaerobic conditions! Remember. oil/natural gas formation Partially decomposed organic matter from past geological eras was converted into either coal, or oil, or gas, which accumulates in porous rocks Oil, i.e. petroleum, and natural gas, form as the result of the decay of marine organisms on the ocean floor. ● Sediments, such as clay and mud, are deposited on top of the organic matter, creating anoxic conditions that prevent decomposition ● As a result of the burial and compaction, the organic material becomes heated and hydrocarbons are formed ● The hydrocarbons form oil and gas, which are forced out of the source rock and accumulate in porous rocks, e.g. sandstone Carbon Source 5: Pollution (Combustion) When organic compounds rich in hydrocarbons are heated in the presence of oxygen, they undergo a combustion reaction. ● This reaction is exergonic, it produces energy, and releases carbon dioxide and water as by products ● The carbon dioxide is typically released into the atmosphere, increasing the concentration of the gas in the air ● For example, complete combustion of propane (refer to chemistry) Combustion Source 1: Fossil Fuels Organic compounds can become rich in hydrocarbons when compacted underground for millions of years ● The heat and pressure over time, triggers a chemical transformation that results in the compaction of the organic matter ● The resulting products of this process are fossil fuels (coal, oil and natural gas) ● Because this geological process takes millions of years to occur, fossil fuels are a non-renewable energy source Combustion Source 2: Biomass An alternative to relying on fuels produced by geological processes is to manufacture fuels from biological processes. You can produce some renewable energy from biomass. ● Living organisms produce hydrocarbons as part of their total biomass, either for use, or as a waste product ● These hydrocarbons can be extracted and purified to produce an alternative fuel source, e.g bioethanol and biodiesel ● Provided new raw materials are provided and waste products are removed, this source of energy is renewable Carbon Fluxes Carbon fluxes describe the rate of exchange of carbon between the various carbon sinks / reservoirs. ● There are 4 main carbon sinks: ○ ○ ○ ○ Lithosphere (earth crust) Hydrosphere (oceans) Atmosphere (air) Biosphere (organisms) The rate at which carbon is exchanged between these reservoirs, or carbon fluxes, depend on the conversion processes involved: ● Photosynthesis - removes carbon dioxide from the atmosphere and fixes it in producers as organic compounds ● Respiration - releases carbon dioxide into the atmosphere when organic compounds are digested in living organisms ● Decomposition - releases carbon products into the air or sediment when organic matter is recycled after the death of an organism ● Gaseous dissolution - the exchange of carbon gases between the ocean and atmosphere ● Lithification - the compaction of carbon-containing sediments into fossils and rocks within the Earth’s crust (e.g. limestone) ● Combustion - releases carbon gases when organic hydrocarbons (coal, oil and gas) are burned as a fuel source It is impossible to directly measure the size of the carbon sinks or the fluxes between them - instead estimates are made. ● Global carbon fluxes are very large, and are therefore measured in gigatonnes ○ 1 gigatonne of carbon = 1 billion metric tonnes ● Because carbon fluxes are large and based on measurements from many different sources, estimates have large uncertainties Fluxes are in red* Estimating Carbon Fluxes Estimating carbon fluxes requires an understanding of the factors that can affect the exchange of carbon between different sinks. ● Some of the main causes for flux change include climate conditions, natural events and human activity ● 1. Climate conditions ○ Rates of photosynthesis will likely be higher in summer seasons, as there is more direct sunlight and longer days ○ Oceanic temperatures also determine how much carbon is stored as dissolved CO2 or as hydrogen bicarbonate ions ○ Climate events like El Nino and La Nina will change the rate of carbon flux between ocean and atmosphere ○ Melting of polar ice caps will result in the decomposition of frozen detritus ● Natural events ○ ● Forest fires can release high levels of carbon dioxide when plants burn, loss of trees also reduces photosynthetic carbon uptake ○ Volcanic eruptions can release carbon compounds from the Earth’s crust into the atmosphere Human Activity ○ Clearing of trees for agricultural purposes (deforestation) will reduce the removal of atmospheric CO2 via photosynthesis ○ Increased numbers of ruminant livestock ( ) will produce higher levels of methane ○ The burning of fossil fuels will release carbon dioxide into the atmosphere 🐮 Application: Analysis of data from air monitoring stations to explain annual fluctuations Atmospheric CO2 concentrations have been measured at the Mauna Loa Observatory (in Hawaii) since 1958 by Charles Keeling. From these continuous and regular measurements a clear pattern of carbon flux can be seen: ● CO2 levels fluctuate annually, lower in the summer months when long days and more light increase photosynthetic rates ● Global CO2 trends will conform to northern hemisphere patterns as it contains more of the planet’s land mass (i.e more trees) ● CO2 levels are steadily increasing year on year since the industrial revolution, due to increased burning of fossil fuels ● Atmospheric CO2 levels are currently at the highest levels recorded since measurements began Data is now being regularly collected at a variety of field stations globally, using standardised measurement techniques ● All station show a clear upward trend in atmospheric CO2 concentrations year on year, with annual fluctuations ● Different monitoring stations may have slightly different trends due to seasonal variations and the distribution of local vegetation Climate Change Greenhouse Gases Greenhouse gases absorb and emit long-wave infrared radiation, thereby trapping and holding heat within the atmosphere. ● Greenhouse gases collectively make up less than 1% of the Earth’s atmosphere The greenhouse gases which have the largest warming effect within the atmosphere are water vapour (clouds) and carbon dioxide ● These 2 are the worst!!! ● ● ● ● Water vapour is created via evaporation of water bodies, like oceans, and transpiration Water vapour is removed via precipitation (rain) Carbon dioxide is made by cell respiration and burning fossil fuel It is removed via photosynthesis and absorption by ocean Other greenhouse gases include methane and nitrogen oxides - these have less impact on the overall warming effect ● Methane is emitted from waterlogged habitats, such as marshes, and landfills!!! It is also a gaseous waste produced by ruminants (cow cow) ● Nitrogen oxides are released naturally by certain bacteria and also emitted in the exhaust by certain vehicles The most abundant greenhouse gas in the atmosphere is water vapour, but is not produced as a product of human activity The greenhouse gases: water vapour, carbon dioxide, methane, nitrogen oxides The impact of a greenhouse gas (warming the atmosphere) There are 2 factors which determine how much of an impact a greenhouse gas will have in warming the atmosphere 1. Ability to absorb long-wave radiation a. Gases that have a greater capacity to absorb long-wave radiation will have a greater warming impact (per molecule) 2. Concentration within the atmosphere a. The greater the concentration of the gas, the greater its warming impact will be within the atmosphere b. The concentration of a gas will be determined by both its rate of persistence and release within the atmosphere The overall impact of a greenhouse gas will be determined by the combination of both these factors ● Methane has a larger capacity to absorb long wave radiation than carbon dioxide, but is significantly less abundant ● Water vapour enters the atmosphere rapidly but only remains for short periods, while carbon dioxide persists for years ● Human activity is increasing the amount of greenhouse gases (except water vapour) and hence increasing their impact The greenhouse effect is naturally occurring but then anthropomorphic activity amplifies it. Carbon Dioxide Concentrations While greenhouse gases occur naturally, man is increasing greenhouse gas emission via a number of activities, including: ● Deforestation - the removal of trees means that less carbon dioxide is removed from the atmosphere via photosynthesis ● Increased farming and agriculture involves land clearing for cattle grazing, also ruminant cattle produce methane The greenhouse gas that is increasing most rapidly in the atmosphere is carbon dioxide, and the main cause is combustion ● When fossil fuels, such as coal, oil and gas, are combusted to release energy, carbon dioxide gas is released as a by-product ● The increased reliance on fossil fuels following the industrial revolution has increased in a ~38% increase in CO2 levels ● There are now efforts to reduce our reliance on fossil fuels by exploiting alternative energy sources, such as solar power Climate Changes Global temperatures and climate patterns are influenced by concentration of greenhouse gases Greenhouse gases play a pivotal role in determining global temperatures and climate patterns due to their capacity to retain heat ● As these gases trap heat, increases in greenhouse gas concentrations should correlate with an increase in global temperature ● Long term weather patterns (climate) may also be influenced by greenhouse gas concentrations Scientists predict that increases in greenhouse gas concentrations will lead to an enhanced greenhouse effect, resulting in: ● More frequent extreme weather conditions (heat waves, cyclones, more powerful tropical storms) ● Some areas to become more drought affected, while other areas become more prone to periods of heavy rainfall ● Changes to circulating ocean currents - which may cause longer EL NINO (warming) and LA NINA (cooling) events Correlations between global temperatures and carbon dioxide concentrations on Earth The link between global temperatures and carbon dioxide concentrations was established by analysing data over a long time period ● Ice cores taken from the Vostok station in antarctica provide evidence of environmental conditions at the time of freezing ● The Vostok ice core is one of the longest drilled, reaching back 420, 000 years and covering the past four glacial cycles ● By analysing the gas bubbles trapped in ice, historical CO2 levels and air temperatures (via oxygen isotopes) can be deduced Data collected from the Vostok ice core demonstrates that: ● There is a strong positive correlation between carbon dioxide concentrations and temperature (↑ CO2 levels ↑ temperature) ● There have been fluctuating cycles of CO2 concentrations which appear to correlate with global warm ages and ice ages ● Current concentrations of CO2 are higher than at any time recorded in the last 400,000 years Industrial revolution The industrial revolution introduced new manufacturing processes which significantly increased mankind’s use of fossil fuels. The burning of fossil fuels releases carbon dioxide as a by product, leading to a steady increase in its atmospheric concentration. When fuel emissions, atmospheric CO2 concentrations and global temperatures are compared, the following trends are revealed: ● There is a strong positive correlation between increasing fossil fuel emissions and rising atmospheric concentrations of CO2 ● Atmospheric CO2 concentrations have increased ~38% since pre-industrial times (1800: ~ 280 ppm ; 2010: ~ 380 ppm) ● About 40% of CO2 emissions have remained in the atmosphere, the rest has been absorbed by carbon sinks (mainly oceans) ● This increase in atmospheric carbon dioxide concentration correlates with an increase in average global temperature ● While correlation doesn't equal causation, there is mounting evidence to suggest that CO2 emissions are linked to global temperature changes (although other factors likely also contribute) Ocean Acidification The oceans are a major carbon sink and absorb roughly a third of all human produced (anthropomorphic) CO2 emissions ● CO2 solubility is temperature dependent, they are more soluble when cooler, so less CO2 will be absorbed as temperature rises When oceans absorb atmospheric CO2 some of it will remain dissolved in a gaseous state but most will be chemically modified ● Carbon dioxide will combine with water to form carbonic acid, which dissociates into hydrogen ions and hydrogen carbonate ● H+ ions will lower the ocean PH (acidification) and will also combine with free carbonate ions to form more hydrogen carbonate ● With less free carbonate ions in the water, marine organisms are less able to produce calcium carbonate via calcification ● Calcium carbonate is used to form the hard exoskeleton of coral and is also present in the shells of certain molluscs ● Hence increasing concentrations of dissolved carbon dioxide threatens the viability of coral reefs and certain molluscs ● You should see above notes^^^ Carbon Dioxide emissions and ocean acidification Rising levels of atmospheric carbon dioxide are causing a decrease in the pH of ocean water (ocean acidification) ● Since the start of the industrial revolution ocean pH has dropped from ~8.2 to ~8.1, which is roughly a 30% increase in acidity ● It is predicted that if current conditions continue, oceanic pH could fall to roughly 7.8 by the turn of the century 2100 The decrease in ocean pH is predicted to threaten the survival of marine organisms that require calcium carbonate ● An increase in the concentration of H+ ions means there are less free carbonate ions available for calcification ● Shells and coral exoskeletons are also likely to begin to dissolve when ocean conditions are more acidic ● Experiments have shown that increasing water acidity correlates with the significant thinning of shells over several weeks ● Corals, sea urchins and shelled molluscs do not exist in regions with high levels of dissolved CO2 (like hydrothermal vents) Consequences of ocean acidification An increase in ocean acidification as a result of elevated anthropomorphic CO2 emissions could have several consequences: ● The disappearance of coral reefs could result in a loss of shoreline protection and habitat, altering coastal ecosystems ● The loss in revenue from tourism and food industries is predicted to cost economies upwards of $1 trillion by 2100 ● Increasing the dissolved CO2 levels in oceans would cause invasive species of algae to flourish (more photosynthesis) Greenhouse Debate Many claims have been made regarding the impact of human activities on climate change - not all are supported by evidence. ● Many arguments are not backed by reliable scientific data or are made by entities with vested interests, such as oil companies Here are some false claims: Claim 1: Climate change has changed in the past and current trends merely reflect the Earth’s natural climatic cycle ● Supporting argument: ○ Data collected from the vostok ice core shows several changes in climate over the last 400, 000 ○ At several points in history, global average temperatures have been warmer than those currently observed ● Counter argument: ○ Climate changes do occur naturally, but usually not as abruptly as what is seen currently ○ When global warming occurred abruptly in the past, it was always highly destructive to life for example mass extinctions ○ Atmospheric CO2 levels positively correlate to average global temperatures and are currently at the highest levels ever recorded Claim 2: Climate change is being caused by solar activity and the effect of greenhouse gas emissions is negligible ● Supporting argument: ○ Temperatures on earth are influenced by the amount of solar radiation from the sun, more radiation = warmer temperatures ○ Warmer temperatures may be caused by an increase in solar irradiance by the sun, as determined by the number of sunspots ● Counter argument: ○ Over the last 35 years the sun has shown a slight cooling trend, however average global temperatures have increased ○ There is no evidence to support a correlation between solar irradiance and current global temperature trends Claim 3: Certain changes in climate conditions cannot be linked to greenhouse gas emissions ● Supporting argument: ○ Global sea levels began to increase before greenhouse gas emissions significantly increased following the industrial revolution ○ Therefore climate changes like rising sea levels are unrelated to greenhouse gas emissions ● Counter argument: ○ ○ ○ The overall pattern of change in sea levels will be influenced by the time over which the data is collected While sea levels did increase preceding the industrial revolution, this rise in sea levels followed a preceding period of decrease The rate at which sea levels have risen in the past 30 years is greater than that seen in the last 200 years Claim 4: Variability between predicted climate change models means that such models are unreliable ● Supporting argument: ○ Three different models of predicted climate change commissioned by the IPCC show variation of more than 5ºC ○ Climate change models are based on assumptions and if those assumptions are false, the predictions will be incorrect ● Counter Argument: ○ The assumptions made by the different models relate to the extent of human activity predicted over the next 100 years ○ Model A1B predicts a continued reliance on fossil fuels while model B1 predicts a reduction in the current use of raw materials ○ All three models still predict an increase in average global temperatures over the next 100 years Claim 5: Increases in greenhouse gas concentrations in the atmosphere will not be enough to cause significant climate change ● Supporting argument: ○ As of 2009, there were only ~39 molecules of carbon dioxide per 100,000 molecules in the atmosphere ○ At our current rate of CO2 emission, it will take mankind another 5 years to raise that level by 1 molecule (to 40 per 100,000) ○ While we may double atmospheric CO2 levels by the end of the century, doubling a small number still produces a small number ● Counter Argument: ○ The reason why carbon dioxide is so important to the environment is because there is so little of it ○ Living things require constant internal environments (homeostasis) – small external changes can have big impacts on viability