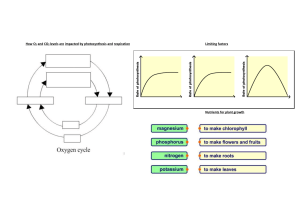
Chapter 2: Essential Chemistry for Biology 1. Consider the radioactive isotope fluorine-18. Fluorine-18 differs from fluorine-19 in A) its atomic mass. B) the number of neutrons found in the nucleus. C) the stability of its nucleus. D) all of the above. 2. The reactive properties (chemical behavior) of an atom mostly depend on the number of A) neutrons found in the nucleus. B) filled electron shells. C) electrons in the outer electron shell of the atom. D) protons in the nucleus. 3. Water molecules form hydrogen bonds because A) the water molecule is polar. B) the oxygen molecule is positively charged. C) the water molecule forms a tetrahedron. D) the hydrogen atoms are negatively charged. 4. Diurnal temperature variation is a terms that describes the difference between a location’s daytime high temperature and nighttime low temperature. Given what you know about how water moderates temperature, which of the following locations would you expect to have the greatest diurnal temperature variation? A) Phoenix, Arizona, with an average humidity of 37% B) Washington, DC, with an average humidity of 61% C) Hong Kong, with an average humidity of 80% 5. This is the general equation for photosynthesis—the process of capturing energy from sunlight and converting it to chemical energy. Which of the following are the reactants of this reaction? A) C6H12O6 and O2 B) CO2 and H2O 6. Earth’s oceans are immense. Small floating plants called phytoplankton contribute to ocean productivity (the rate of photosynthesis). If ocean productivity were to increase, what would you predict would happen to global carbon dioxide levels? Hint: Use the reaction above. A) CO2 levels should also go up. B) CO2 levels should go down. C) CO2 levels should remain constant.