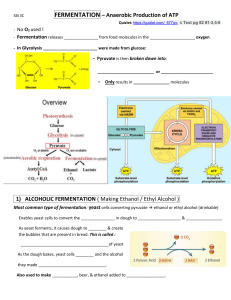
Dean Cutrer March 25th, 2020 Fermentation Introduction: One of the principles of Biology is that all living things use energy. Plants produce chemical energy in a process called photosynthesis. Plants are autotrophs. They make their own organic molecules from sunlight and inorganic sources. Animals and many other organisms obtain our energy by eating or breaking down already formed organic molecules. These organisms are heterotrophs. All cells use the nucleotide adenosine triphosphate (ATP) to power most cellular processes. The process of converting chemical energy of organic molecules into ATP is called cellular respiration. There are various kinds of cellular respiration. The most successful type is aerobic respiration. Aerobic respiration uses oxygen and is the type of respiration used by most large organisms. Aerobic respiration breaks down glucose to form large amounts of ATP in four stages. The first is glycolysis which is carried out in the cytoplasm or cytosol of most living things and converts glucose in to two molecules of pyruvate. The second stage is the breakdown of pyruvate to form acetyl-CoA followed by the third stage which is citric acid cycle or Krebs cycle. The Citric acid cycle happens in the mitochondria of a eukaryotic cell. During these reactions, high-energy electrons are transferred in a series of oxidation —reduction reactions. These electrons are then used in the fourth stage of aerobic respiration, electron transport which produces most of the ATP made. At the end of the electron transport, chain oxygen accepts the electrons and is reduced. When oxygen is limiting many organisms have other options for respiration. One of these anaerobic pathways is fermentation. In fermentative pathways glycolysis is still used to produce pyruvate. The pyruvate can then be converted to either lactic acid or carbon dioxide and ethanol. This process will produce a small amount of ATP. For each molecule of glucose that goes through fermentation two ATPs are produced. Lactic acid fermentation occurs in some human cells such as muscle tissue. Microorganisms such as yeast often use acholic fermentation which is important in baking and in the brewing of beer and wine making. Purpose/Objective: This experiment is designed to examine the effect of different variable on the rate of fermentation. The yeast in this experiment will ferment their carbon source to form CO2 and ethanol. My hypothesis is that there will be a increase in the yeast change for week two compared to the week one yeast. Null Hypothesis: Nothing will change Materials: • Fermentation tubes • Balance • Yeast • Sugar • Water • Weigh boats and spatulas • Ruler (if fermentation tubes not graduated) • Stir rod Methods/Procedure: Week 1: 1. Each group will weigh out 0.2 g of yeast. 2. Weigh out 0.5 g of sucrose. 3. Add yeast and sucrose to 10 mL of warm water and mix with a stirring rod. 4. Pour yeast and sugar mix into fermentation tube. 5. Cover the opening of the fermentation tube and invert the tube so the long portion of the tube fills with the solution. 6. After ten minutes measure the distance from the top of the fermentation tube to the top of the solution and record the distance in centimeters or millimeters. 7. Repeat steps 5 and 6 every ten minutes until six measurements are made or until no more measurements are possible. 8. When finished measure the width of the tail portion of the fermentation tube. 9. Calculate the radius or the tail portion of the fermentation tube. (The radius is 1/2 the diameter or width) 10. Use the formula 7tr2 to calculate the cross-sectional area of the fermentation tube. 11. For each ten-minute interval, multiply the distance recorded by the cross-sectional area to find the volume of the carbon dioxide. 12. Clean up. Week 2: Repeat with different variables from TA Results: Week 1 10 min 20 min Distance 0 cm 0 cm Volume 0 0 cm^3 cm^3 Week 2 10 min Distance 2.4 cm Volume 2.7 cm^3 30 min 1.0 cm 1.1 cm^3 20 30 min min 6.0 cm 8.5 cm 6.8 cm^3 9.6 cm^3 Week 1: Average/Mean: 0.33333333 Median: 0 Mode:0 STND: 0.57735027 P.Value: 0.04230802 Week 2: Average/Mean: 5.63333333 Median: 6 Mode: N/A STND: 3.0664855 P.Value: 0.04230802 Conclusion: After conducting the experiment, the P.value is .04 which means we can reject out null hypothesis. My hypothesis stating that the yeast for week two will have a higher increase than week one was correct. Yeast Fermentation Distance in CM 10 8 6 4 2 0 1 2 3 Time in Minutes Per (10) Week 1 Week 2