Isotopes Worksheet: Definition, Calculations, and Tables
advertisement

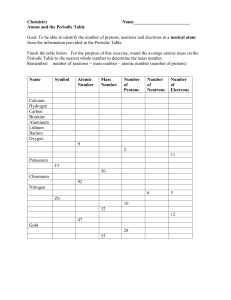
Isotopes Isotopes are a version of the same element but they have a different mass number. The mass number tells us the number of protons added to the number of neutrons. As the atomic number is the same, it must be the number of neutrons that is different. For example there are two common isotopes for Chlorine: 35 17𝐶𝑙 37 17𝐶𝑙 We take an average of the number numbers to come up with the mass number which is in the periodic table of 35.5. Task 1 Use this information to write a simple definition for an isotope. ...................................................................................................................... ...................................................................................................................... Task 2 Calculate the number of neutrons for each of the isotopes below. Remember we do this by subtracting the atomic number from the mass number: Isotope mass number atomic number chlorine 35 35 17 chlorine 37 37 17 bromine 79 79 35 bromine 81 81 35 number of neutrons Task 3 Look at the table below and use your periodic table to complete the missing numbers. Find another example of an element that has an isotope and fill in the last two rows. atomic number mass number 1 1 1 2 1 2 H H 12 C 12 number of protons number of neutrons C 7 Na 12 23 Na O O © www.teachitscience.co.uk 2016 11 24 16 18 electronic structure 6 13 24 number of electrons 2,6 18 25799 Page 1 of 2 Isotopes Answers Task 2 Isotope mass number atomic number number of neutrons chlorine 35 35 17 18 chlorine 37 37 17 20 bromine 79 79 35 44 bromine 81 81 35 46 Task 3 atomic number mass number number of protons number of neutrons number of electrons electronic structure 1H 1 1 1 0 1 1 2 1 2 1 1 1 1 12 6 12 6 6 6 2,4 13 C 6 13 6 7 6 2,4 23 Na 11 23 11 12 11 2,8,1 24 11 24 11 13 11 2,8,1 16 8 16 8 8 8 2,6 18 8 18 8 10 8 2,6 H C Na O O © www.teachitscience.co.uk 2016 25799 Page 2 of 2