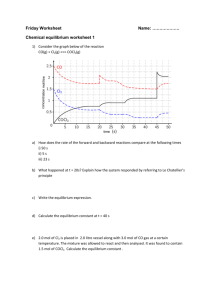
CHEMICAL EQUILIBRIUM (ii) PRACTICE TEST QUESTIONS CHEM 40S [ the “**” indicates that the question would actually be a long answer question if it was placed on a real test] 1. What is the equilibrium concentration of lead (II) ions [Pb2+], in a saturated solution of lead (II) chloride, given the concentration of all the chloride ions [Cl-­] at solubility equilibrium is 0.032 mol/L. Note: the initial concentrations of both the lead and chloride ions were zero. PbCl2(s) ⇌ Pb2+(aq) + 2Cl-­(aq) a. 0.0051 M b. 0.016 M c. 0.032 M d. 0.064 M e. 0.098 M 2. **What is the Ksp of lead(II) chloride (PbCl2(s)), in a saturated solution given the concentration of all the chloride ions [Cl-­] at solubility equilibrium is 0.032 mol/L. Note: the initial concentrations of both the lead and chloride ions were zero. PbCl2(s) ⇌ Pb2+(aq) + 2Cl-­(aq) a. b. c. d. e. 3. 5.1x10-­4 4.8x10-­3 3.9x10-­5 6.2x10-­2 1.6x10-­5 **Calculate the molar solubility of Lead (II) fluoride (PbF2) given the solubility product constant for the reaction is 3.7x10-­8. PbF2(s) ⇌ Pb2+(aq) + 2F-­(aq) a. b. c. d. 8.5x10-­1 M 3.3x10-­3 M 2.1x10-­3 M 1.4x10-­4 M Page 1 of 14 4. 5. An equilibrium that strongly favours products has __________. a. A value of K <<1 b. A value of K >>1 c. A value of Q <<1 d. A value of Q>> 1 e. K=Q 6. A chemical system that has to flow to the left to re-­establish equilibrium will have __________________. a. A value of Q < K b. A value of Q = K c. A value of Q > K d. It is not possible for an equilibrium to flow left 7. A chemical equilibrium that results in the production of a precipitate has _____________. a. A value of Ksp <Qsp b. A value of Ksp > Qsp c. A value of Qsp = 1 d. Ksp= Qsp If the reaction quotient Q has a smaller value than the related equilibrium constant, K , ______________. a. The reaction is at equilibrium b. The reaction is not at equilibrium, and will make more products at the expense of reactants c. The reaction is not at equilibrium, and will make more reactants at the expense of products d. The value of K will decrease until it is equal to Q 8. **Calculate the g/100mL solubility of Lead (II) fluoride (PbF2) given the solubility product constant for the reaction is 3.7x10-­8. PbF2(s) ⇌ Pb2+(aq) + 2F-­(aq) a. 0.0021 g/100mL b. 0.021 g/100mL c. 0.51 g/100ml d. 0.051 g/100mL e. 5.1 g/100mL Page 2 of 14 9. 10. 11. 12. If the reaction quotient Q has a larger value than the related equilibrium constant, K, ______________. a. The reaction is at equilibrium b. The reaction is not at equilibrium, and will make more products at the expense of reactants c. The reaction is not at equilibrium, and will make more reactants at the expense of products d. The value of K will decrease until it is equal to Q If the equilibrium is established by initially adding 0.10 mol each of A and B to a 1L container, then which of the following must be true once the mixture achieves equilibrium? A + B ⇌ 2C K = 324 a. [A] = [B] b. [A] < [B] c. [A] > [B] d. [A] = [B] = [C] e. [B] = 2[C] **If the equilibrium is established by initially adding 0.10 mol each of A and B to a 1L container, then which of the following must be true once the mixture achieves equilibrium? A + B ⇌ 2C K = 324 a. [A] = 0.09 M b. [B] = 0.09 M c. [C] = 0.01 M d. [A] = 0.01 M When the system A + B ⇌ C + D is at equilibrium, (a) the sum of the concentrations of A and B must equal the sum of the concentrations of C and D. (b) the forward reaction has stopped. (c) both the forward and the reverse reactions have stopped. (d) the reverse reaction has stopped. (e) neither the forward nor the reverse reaction has stopped. Page 3 of 14 13. 2SO3(g) ⇌ 2SO2(g) + O2(g) The conventional equilibrium constant expression (Kc) for the system as described by the above equation is: (a) [SO2]2/[SO3] (b) [SO2]2[O2]/[SO3]2 (c) [SO3]2/[SO3]2[O2] (d) [SO2][O2] (e) none of these 14. Consider the following reversible reaction. In a 3.00 liter container, the following amounts are found in equilibrium at 400 oC: 0.0420 mole N2, 0.516 mole H2 and 0.0357 mole NH3. Evaluate Kc. N2(g) + 3H2(g) ⇌ 2NH3(g) (a) 0.202 (b) 1.99 (c) 16.0 (d) 4.94 (e) 0.503 15. If the equilibrium constant for the reaction A + 2B ⇌ C + 5/2 D has a value of 4.0, what is the value of the equilibrium constant for the reaction 2C + 5D ⇌ 2A + 4B at the same temperature? (a) 0.25 (b) 0.063 (c) 2.0 (d) 8.0 (e) 16 Page 4 of 14 16. **At 445oC, Kc for the following reaction is 0.020. 2HI(g) ⇌ H2(g) + I2(g) A mixture of H2, I2, and HI in a vessel at 445oC has the following concentrations: [HI] = 2.0 M, [H2] = 0.50 M and [I2] = 0.10 M. Which one of the following statements concerning the reaction quotient, Qc, is TRUE for the above system? (a) Qc = Kc;; the system is at equilibrium. (b) Qc is less than Kc;; more H2 and I2 will be produced. (c) Qc is less than Kc;; more HI will be produced. (d) Qc is greater than Kc;; more H2 and I2 will be produced. (e) Qc is greater than Kc;; more HI will be produced. 17. **Nitrosyl chloride, NOCl, dissociates on heating as shown below. When a 1.50 gram sample of pure NOCl is heated at 350oC in a volume of 1.00 liter, the percent dissociation is found to be 57.2%. Calculate Kc for the reaction as written. NOCl(g) ⇌ NO(g) + 1/2 Cl2(g) (a) 0.876 (b) 9.26 (c) 0.107 (d) 1.75 x 10-­4 (e) 0.0421 18. **A quantity of HI was sealed in a tube, heated to 425oC and held at this temperature until equilibrium was reached. The concentration of HI in the tube at equilibrium was found to be 0.0706 mol/L. Calculate the equilibrium concentration of H2 (and I2). For the gas-­phase reaction, H2 + I2⇌ 2HI Kc = 54.6 at 425oC (a) 9.55 x 10-­3 M (b) 1.17 x 10-­3 M (c) 1.85 x 10-­4 M (d) 4.78 x 10-­3 M (e) 2.34 x 10-­3 M Page 5 of 14 19. **Consider the reaction: N2(g) + O2(g) ⇌ 2NO(g) Kc = 0.10 at 2000oC Starting with initial concentrations of 0.040 mol/L of N2 and 0.040 mol/L of O2, calculate the equilibrium concentration of NO in mol/L (a) 0.0055 mol/L (b) 0.0096 mol/L (c) 0.011 mol/L (d) 0.080 mol/L (e) 0.10 mol/L 20. **Kc = 0.040 for the system below at 450oC. If a reaction is initiated with 0.40 mole of Cl2 and 0.40 mole of PCl3 in a 2.0 liter container, what is the equilibrium concentration of Cl2 in the same system? PCl5(g) ⇌ PCl3(g) + Cl2(g) (a) 0.07 M (b) 0.16 M (c) 0.11 M (d) 0.04 M (e) 0.26 M 21. **The reversible reaction: 2SO2(g) + O2(g) ⇌ 2SO3(g) has come to equilibrium in a vessel of specific volume at a given temperature. Before the reaction began, the concentrations of the reactants were 0.060 mol/L of SO2 and 0.050 mol/L of O2. After equilibrium is reached, the concentration of SO3 is 0.040 mol/L. What is the equilibrium concentration of O2? (a) 0.010 M (b) 0.020 M (c) 0.030 M (d) 0.040 M (e) none of these Page 6 of 14 22. Consider the gas-­phase equilibrium system represented by the equation: 2H2O(g) ⇌ 2H2(g) + O2(g) Given that the forward reaction (the conversion of "left-­hand" species to "right-­hand" species) is endothermic, which of the following changes will decrease the equilibrium amount of H2O? (a) adding more oxygen (b) adding a solid phase catalyst (c) decreasing the volume of the container (the total pressure increases) (d) increasing the temperature at constant pressure (e) adding He gas 23. The conventional equilibrium constant expression (Kc) for the system below is: 2ICl(s) ⇌ I2(s) + Cl2(g) (a) [I2][Cl2]/[ICl]2 (b) [I2][Cl2]/2[ICl] (c) [Cl2] (d) ([I2] + [Cl2])/2[ICl] (e) [Cl2]/[ICl]2 24. Consider the equilibrium system: 2ICl(s) I2(s) + Cl2(g) Which of the following changes will increase the total amount of of Cl2 that can be produced? (a) removing some of the I2(s) (b) adding more ICl(s) (c) removing the Cl2 as it is formed (d) decreasing the volume of the container (e) all of the above Page 7 of 14 25. At equilibrium, a 1.0 liter container was found to contain 0.20 moles of A, 0.20 moles of B, 0.40 moles of C and 0.40 mole of D. If 0.10 moles of A and 0.10 moles of B are added to this system, what will be the new equilibrium concentration of A? A(g) + B(g) C(g) + D(g) (a) 0.37 mol/L (b) 0.47 mol/L (c) 0.87 mol/L (d) 0.23 mol/L (e) 0.15 mol/L 26. Consider the following system in a 1.00 L container: A(g) + B(g) 2C(g) The equilibrium concentrations at 200oC were determined to be: [A] = 0.200 M [B] = 3.00 M [C] = 0.500 M How many moles of A must be added to increase the concentration of C to 0.700 M at 200oC? (a) 0.225 mol (b) 0.305 mol (c) 0.417 mol (d) 0.610 mol (e) 0.700 mol 27. Consider the reversible reaction at equilibrium at 392oC: 2A(g) + B(g) C(g) The partial pressures are found to be: A: 6.70 atm, B: 10.1 atm, C: 3.60 atm. Evaluate Kp for this reaction. (a) 7.94 x 10-­3 (b) 0.146 (c) 0.0532 (d) 54.5 (e) 121 Page 8 of 14 28. Kc = 0.040 for the system below at 450oC: PCl5(g) PCl3(g) + Cl2(g) Evaluate Kp for the reaction at 450oC. (a) 0.40 (b) 0.64 (c) 2.4 (d) 0.052 (e) 6.7 x 10-­4 29. For a specific reaction, which of the following statements can be made about K, the equilibrium constant? (a) It always remains the same at different reaction conditions. (b) It increases if the concentration of one of the products is increased. (c) It changes with changes in the temperature. (d) It increases if the concentration of one of the reactants is increased. (e) It may be changed by the addition of a catalyst. 30. **Methoxymethane, CH3OCH3, is used as an environmentally friendly propellant in spray cans. It can be made from methanol according to the following equation: 2CH3OH(g) ⇌ CH3OCH3(g) + H2O(g) ΔH = –24 kJ The equilibrium constant, Kc, for this reaction at 350 °C is 5.74. A 1.00 L vessel at 350 °C initially contains 0.500 mole of CH3OH(g), 0.100 mole of CH3OCH3(g) and 0.700 mole of H2O(g). Calculate Q of the system and decide how the system will respond. a. The system will respond by producing less products and more reactants b. The system will respond by producing more products and less reactants c. The system will respond by producing more products and more reactants d. The system will respond by producing less products and less reactants Page 9 of 14 31. **PCl5(g) decomposes to form PCl3(g) and Cl2(g) according to the equation: PCl5(g) ⇌ PCl3(g) + Cl2(g) Four different flasks, A, B, C and D, at the same temperature, contain a mixture of PCl5(g), PCl3(g) and Cl2(g). The concentration, in mol dm–3, of these components in each of the flasks is shown in the multiple choice options below. In three of the four flasks, the mixture of gases is at equilibrium (producing the same equilibrium expression value). In which one is the mixture of gases NOT at equilibrium? [PCl5(g)] [PCl3(g)] [Cl2(g)] A 0.10 0.40 0.10 B 0.15 0.20 0.30 C 0.20 0.30 0.15 D 0.30 0.60 0.20 32. **Consider the following reaction: 2NOCl(g) ⇌ 2NO(g) + Cl2(g) Initially, some NOCl(g) was placed in a 1.00 L container. At equilibrium, there were 0.860 mole of NOCl(g), 0.0300 mole of NO(g) and 0.0150 mole of Cl2(g). How many moles of NOCl(g) were initially added to the container? a. 0.785 mole b. 0.815 mole c. 0.890 mole d. 0.905 mole 33. **Consider the following reaction: 2SO2(g) + O2(g) ⇌ 2SO3(g) Kc = 4.00 In an experiment, 0.600 mole of SO2(g), 0.300 mole of O2(g) and 0.600 mole of SO3(g) are placed in a 1.00 L container. The system is allowed to reach equilibrium. Which of the following are correct as the system approaches equilibrium? (1) Concentration of SO2(g) increases. (2) Concentration of O2(g) decreases. (3) Concentration of SO3(g) increases. a. (1) and (2) only b. (1) and (3) only c. (2) and (3) only d. (1), (2) and (3) Page 10 of 14 34. 35. Identify the LEAST soluble sulphide. a) HgS b) PbS c) FeS d) MnS Ksp = 1.6 x 10-­54 Ksp = 7.0 x 10-­29 Ksp = 3.7 x 10-­19 Ksp = 2.3 x 10-­13 Which of the following salts has the GREATEST solubility? Copper (I) chloride Silver chloride Barium carbonate Lead (II) iodide 36. Given equal amounts of [0.10 mol] of nitrogen dioxide and dinitrogen tetraoxide are placed in a 1.0 L at 55oC with a K=1.15. N2O4 (g) ⇌ 2NO2 (g) Determine the “C”hange of concentration of nitrogen dioxide (NO2) that would be placed in the I.C.E. chart. a. -­2x b. +2x c. –x2 d. +x2 37. The solubility product constant (Ksp) is used . . . a. Whenever a solution is involved the chemical reaction. b. Whenever the compounds are soluble or very soluble c. Whenever the solution is saturated with the compounds in question d. Whenever there is a homogenous equilibrium e. All of the above 38. **Using the SOLUBILITY CONSTANTS and periodic table with ion list attached to the back of the test, predict if a precipitate will form if 15 mL of 0.020 M Lead(II) nitrate is mixed with 25 mL of 0.10 M sodium chloride. a. Yes a precipitate of Lead (II) chloride will form b. Yes a precipitate of Sodium nitrate will form c. No precipitates will form d. There is not enough information provided to tell a. b. c. d. Page 11 of 14 Answers: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. b e c d b c a b c a d e b b b b c a c a c d c c d b a c c b c c c a d b c a Page 12 of 14 Solubility Product Constants (KSP) Solubility Product Constants at 25oC Name Formula barium carbonate barium chromate barium sulfate calcium carbonate calcium oxalate calcium phosphate calcium sulfate copper(l) chloride copper(l) iodide copper(ii) iodate copper(ll) sulfide iron(II) hydroxide iron(ll) sulfide iron(llI) hydroxide lead(ll) bromide lead(Il) chlonde lead(ll) iodate lead(ll) iodide lead(ll) sulfate magnesium carbonate magnesium fluoride magnesium hydroxide mercury(l) chloride silver bromate silver bromide silver carbonate silver chloride silver chromate silver iodate silver iodide strontium carbonate strontium fluoride strontium sulfate zinc hydroxide zinc sulfide BaCO3(S) BaCrO4(s) BaSO4(s) CaCO3(s) CaC204(s); CaOOCCOO(s) Ca3(PO4)2(s) CaSO4(s) CuCl(s) Cul Cu(lO3)2(s) CuS(s) Fe(OH)2(s) FeS(s) Fe(OH)3(s) PbBr2(s) PbCl2(s) Pb(lO3)2(s) Pbl2(s) PbSO4(s) MgCO3(s) MgF2(s) Mg(OH)2(s) Hg2Cl2(s) AgBrO3(s) AgBr(s) Ag2CO3(s) AgCl(s) Ag2CrO4(s) AglO3(s) AgI(s) SrCO3(s) SrF2(s) SrSO4(s) Zn(OH)2(s) ZnS(s) Values in this table are taken from The CRC Handbook of Chemistry and Physics, 76th Edition. Page 13 of 14 Ksp 2.6 x 10-9 1.2 x 10-10 1.1 x 10-10 5.0 x 10-9 2.3 x 10-9 2.1 x 10-33 7.1 x 10-5 1.7 x 10-7 1.3 x 10-12 6.9 x 10-8 6.0 x 10-37 4.9 x 10-17 6.0 x 10-19 2.6 x10-39 6.6 x 10-6 1.2 x 10-5 3.7 x 10-13 8.5 x 10-9 1.8 x 10-8 6.8 x 10-6 6.4 x 10-9 5.6 x 10-12 1.5 x 10-18 5.3 x 10-5 5.4 x 10-13 8.5 x 10-12 1.8 x 10-10 1.1 x 10-12 3.2 x 10-8 8.5 x 10-17 5.6 x 10-10 4.3 x 10-9 3.4 x 10-7 7.7 x 10-17 2.0 x 10-25 Page 14 of 14