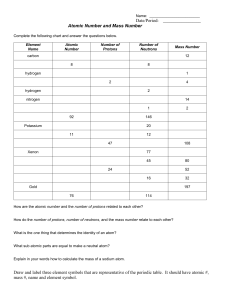
DO NOW TUESDAY • Quietly sit down and begin work on your Physical and Chemical Properties and Changes quiz. • When finished, turn your quiz in at the side cart and pick up a half sheet of paper. • Write everything you know about atoms on this sheet. •Today’s PLAN –To differentiate between the subatomic particles of an atom: protons, neutrons, and electrons. –To explain the structure of the nucleus. –To discuss what the atomic mass number and atomic number represent concerning the atoms of an element. •Todays DO •We will complete foldables about the atom, atomic number, and atomic mass number as notes to put in our binders. •We will practice identifying the atomic number and atomic mass number. Any practice that is not completed during class will be HOMEWORK. Atoms Atoms • Atoms are the basic building blocks of all matter. • EVERYTHING on Earth is made of atoms…even the air and your body. e- Atoms are made of three particles: e- • Protons (+) +N + + N +N • Neutrons (N) e- • Electrons (e-) Electron Proton Neutron Protons • Protons • Located in the nucleus • Have a positive charge • Have a mass of one N Neutrons Neutrons • Located in the nucleus • Have no charge • Have a mass of one –similar to the proton N N N Electrons Electrons e- e- • have a negative charge • orbit the nucleus of the atom • are very small (have basically NO mass) • in a neutral atom, there are the same number of protons and electrons Atom Structure Neutron - Proton Electron + + - What force holds all the parts of an atom together? • It is the electromagnetic force of attraction between the positive protons in the nucleus and the negative electrons orbiting around the nucleus that holds the atom together. Lets take a look deep inside Lets take a look deep inside Lets take a look deep inside Lets take a look deep inside + + This is a Proton. + Charge Mass of ONE Identifies the atom + This is a Proton. + Charge N Mass of ONE Identifies the atom + This is a Proton. + Charge N Mass of ONE Identifies the atom e+ This is a Proton. + Charge N This is a Neutron No Charge Mass of One Mass of ONE Identifies the atom This is an Electron. - charge e- No Mass + This is a Proton. + Charge N This is a Neutron. No Charge Mass of ONE Mass of ONE Identifies the atom Atomic Number Elements contain one or more of the same type of atom! All known elements can be found on the periodic table. • Elements can be identified by their atomic number. • The atomic number is the number of PROTONS in the atoms of an element. • It can be used like a social security number for people. • It is used to IDENTIFY the element from the Periodic Table. Example: An element with 6 protons has an atomic number of 6 and is the element Carbon from the Periodic Table. Now You Try • Identify the element and tell how many protons it has: 1) Atomic number 7 Nitrogen: 7 protons 2) Atomic Number 20 Calcium: 20 protons • Identify the element and give its atomic number. 3) 15 protons 4) 4 protons Phosphorus: Atomic number 15 Helium: Atomic Number 4 • Give the atomic number and number of protons. 5) Argon Atomic number 18; 18 protons 6) Sulfur Atomic number 16; 16 protons Atomic Mass Number • The atomic mass number includes the number of protons and neutrons, since they are the two largest particles in the atom. • Since they are both located in the nucleus, the mass of the atom is located in the nucleus. Atomic Mass Number = protons + neutrons Using the Periodic Table Using the Periodic Table 2 Using the Periodic Table 2 Using the Periodic Table Atomic Numberidentifies the element. (also the number of protons) 2 Using the Periodic Table Atomic Numberidentifies the element. (also the number of protons) 2 He Using the Periodic Table Atomic Numberidentifies the element. (also the number of protons) 2 He Using the Periodic Table Atomic Numberidentifies the element. (also the number of protons) Element symbolgives the name of the element. 2 He Using the Periodic Table Atomic Numberidentifies the element. (also the number of protons) Element symbolgives the name of the element. 2 He 4 Using the Periodic Table Atomic Numberidentifies the element. (also the number of protons) Element symbolgives the name of the element. 2 He 4 Using the Periodic Table Atomic Numberidentifies the element. (also the number of protons) Element symbolgives the name of the element. Atomic mass numberThe number of protons + neutrons. 2 He 4 Using the Periodic Table Atomic Numberidentifies the element. (also the number of protons) Element symbolgives the name of the element. Atomic mass numberThe number of protons + neutrons. 2 He 4 Now You Try For the following pictures, give the name of the element, its atomic number, number of protons, and atomic mass number. Beryllium Atomic number 4 4 protons Atomic mass number 9 Sodium Atomic number 11 11 protons Atomic mass number 23 Oxygen Atomic number 8 8 protons Atomic Mass Number 16 Compounds contain more than one type of atom! Example of organic compound (a compound with carbon and hydrogen atoms): Methane (natural gas) – CH4 (1 atom of carbon and four atoms of hydrogen) Example of inorganic compound (a compound without carbon and hydrogen atoms): Water – H2O (2 atoms of hydrogen and one atom of oxygen) - + An ion is an atom or group of atoms with a positive or negative charge!! A particle with a neutral charge has the same number of protons and electrons. An ion does not have the same number of electrons and protons. Examples of ions: • H+ - A hydrogen atom that is missing one electron. The atom has one more proton than electron, and must have a positive charge. •CO32- - Carbonate has two more electrons than protons