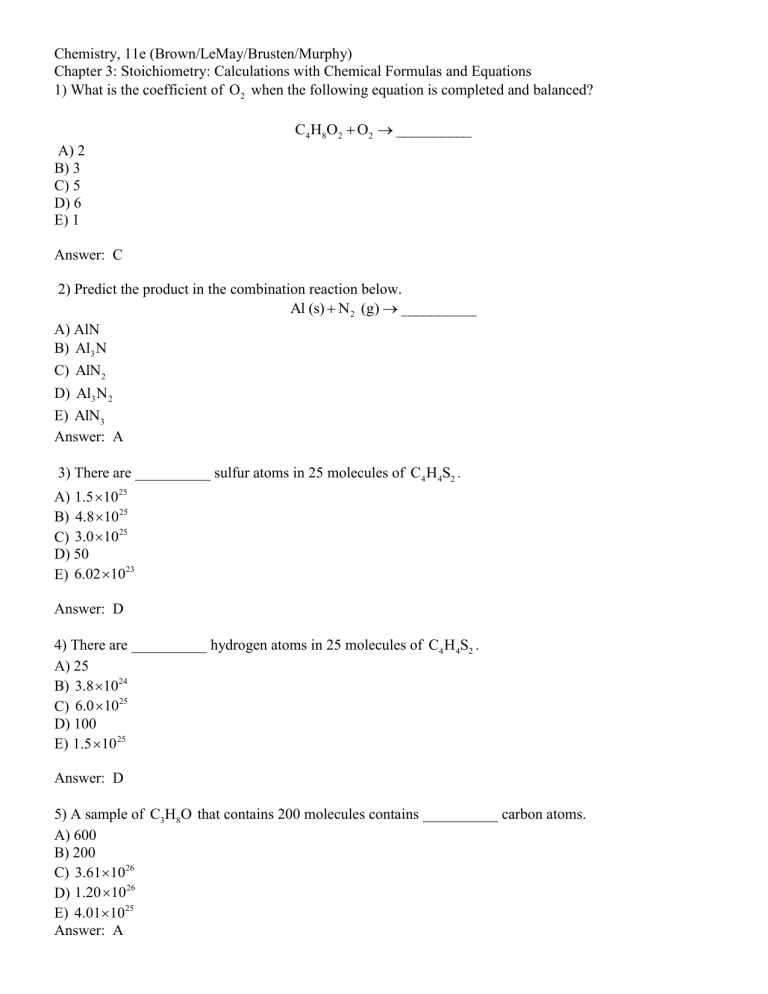
Chemistry, 11e (Brown/LeMay/Brusten/Murphy) Chapter 3: Stoichiometry: Calculations with Chemical Formulas and Equations 1) What is the coefficient of O2 when the following equation is completed and balanced? C4 H8O2 O2 __________ A) 2 B) 3 C) 5 D) 6 E) 1 Answer: C 2) Predict the product in the combination reaction below. Al (s) N2 (g) __________ A) AlN B) Al3 N C) AlN2 D) Al3 N 2 E) AlN3 Answer: A 3) There are __________ sulfur atoms in 25 molecules of C4 H 4S2 . A) 1.5 1025 B) 4.8 1025 C) 3.0 1025 D) 50 E) 6.02 1023 Answer: D 4) There are __________ hydrogen atoms in 25 molecules of C4 H 4S2 . A) 25 B) 3.8 1024 C) 6.0 1025 D) 100 E) 1.5 1025 Answer: D 5) A sample of C3H8O that contains 200 molecules contains __________ carbon atoms. A) 600 B) 200 C) 3.611026 D) 1.20 1026 E) 4.011025 Answer: A Chemistry, 11e (Brown/LeMay/Brusten/Murphy) Chapter 3: Stoichiometry: Calculations with Chemical Formulas and Equations 6) How many grams of hydrogen are in 46 g of CH4O ? A) 5.8 B) 1.5 C) 2.8 D) 0.36 E) 184 Answer: A 7) How many grams of oxygen are in 65 g of C2 H 2O2 ? A) 18 B) 29 C) 9.0 D) 36 E) 130 Answer: D 8) A 22.5-g sample of ammonium carbonate contains __________ mol of ammonium ions. A) 0.468 B) 0.288 C) 0.234 D) 2.14 E) 3.47 Answer: A 9) What is the empirical formula of a compound that contains 27.0% S, 13.4% O, and 59.6% Cl by mass? A) SOCl B) SOCl2 C) S2OCl D) SO2Cl E) ClSO4 Answer: B 10) A compound is composed of only C, H, and O. The combustion of a 0.519-g sample of the compound yields 1.24 g of CO2 and 0.255 g of H 2 O . What is the empirical formula of the compound? A) C6 H 6O B) C3H3O C) CH3O D) C2 H 6O5 E) C2 H6O2 Answer: B Chemistry, 11e (Brown/LeMay/Brusten/Murphy) Chapter 3: Stoichiometry: Calculations with Chemical Formulas and Equations 11) Combustion of a 1.031-g sample of a compound containing only carbon, hydrogen, and oxygen produced 2.265 g of CO2 and 1.236 g of H 2 O . What is the empirical formula of the compound? A) C3H8O B) C3H5O C) C6 H16O2 D) C3H9O3 E) C3H 6 O3 Answer: A 12) Combustion of a 0.9835-g sample of a compound containing only carbon, hydrogen, and oxygen produced 1.900 g of CO2 and 1.070 g of H 2 O . What is the empirical formula of the compound? A) C2 H5O B) C4 H10O2 C) C4 H11O2 D) C4 H10O E) C2 H5O2 Answer: C 13) Magnesium and nitrogen react in a combination reaction to produce magnesium nitride: In a particular experiment, a 9.27-g sample of N 2 reacts completely. The mass of Mg consumed is __________ g. A) 8.04 B) 24.1 C) 16.1 D) 0.92 E) 13.9 Answer: B 14) The combustion of ammonia in the presence of excess oxygen yields NO 2 and H 2 O : The combustion of 28.8 g of ammonia consumes __________ g of oxygen. A) 94.7 B) 54.1 C) 108 D) 15.3 E) 28.8 Answer: A Chemistry, 11e (Brown/LeMay/Brusten/Murphy) Chapter 3: Stoichiometry: Calculations with Chemical Formulas and Equations 15) Lead (II) carbonate decomposes to give lead (II) oxide and carbon dioxide: How many grams of lead (II) oxide will be produced by the decomposition of 2.50 g of lead (II) carbonate? A) 0.41 B) 2.50 C) 0.00936 D) 2.09 E) 2.61 Answer: D 16) What mass in grams of hydrogen is produced by the reaction of 4.73 g of magnesium with 1.83 g of water? A) 0.102 B) 0.0162 C) 0.0485 D) 0.219 E) 0.204 Answer: A 17) Silver(I) nitrate and aluminum chloride react with each other by exchanging anions: What mass in grams of AgCl is produced when 4.22 g of AgNO3 react with 7.73 g of AlCl3 ? A) 17.6 B) 4.22 C) 24.9 D) 3.56 E) 11.9 Answer: D 18) How many moles of magnesium oxide are produced by the reaction of 3.82 g of magnesium nitride with 7.73 g of water? A) 0.114 B) 0.0378 C) 0.429 D) 0.0756 E) 4.57 Answer: A 19) A 3.82-g sample of magnesium nitride is reacted with 7.73 g of water. The yield of MgO is 3.60 g. What is the percent yield in the reaction? A) 94.5 B) 78.7 C) 46.6 D) 49.4 E) 99.9 Answer: B Chemistry, 11e (Brown/LeMay/Brusten/Murphy) Chapter 3: Stoichiometry: Calculations with Chemical Formulas and Equations 20) Solid aluminum and gaseous oxygen react in a combination reaction to produce aluminum oxide: The maximum amount of Al2 O3 that can be produced from 2.5 g of Al and 2.5 g of O2 is __________ g. A) 9.4 B) 7.4 C) 4.7 D) 5.3 E) 5.0 Answer: C 21) Sulfur and fluorine react in a combination reaction to produce sulfur hexafluoride: The maximum amount of SF6 that can be produced from the reaction of 3.5 g of sulfur with 4.5 g of fluorine is __________ g. A) 12 B) 3.2 C) 5.8 D) 16 E) 8.0 Answer: C 22) Solid aluminum and gaseous oxygen react in a combination reaction to produce aluminum oxide: In a particular experiment, the reaction of 2.5 g of Al with 2.5 g of O2 produced 3.5 g of Al2 O3 . The % yield of the reaction is __________. A) 74 B) 37 C) 47 D) 66 E) 26 Answer: A 23) Which one of the following substances is the product of this combination reaction? Al (s) I2 (s) A) AlI 2 B) AlI C) AlI3 D) Al2 I3 E) Al3I2 Answer: C Chemistry, 11e (Brown/LeMay/Brusten/Murphy) Chapter 3: Stoichiometry: Calculations with Chemical Formulas and Equations 24) Gaseous argon has a density of 1.40 g/L at standard conditions. How many argon atoms are in 1.00 L of argon gas at standard conditions? A) 4.76 1022 B) 3.43 10 25 C) 2.1110 22 D) 1.59 10 25 E) 6.02 1023 Answer: C 25) A nitrogen oxide is 63.65% by mass nitrogen. The molecular formula could be __________. A) NO B) NO 2 C) N 2 O D) N 2 O4 E) either NO 2 or N 2 O4 Answer: C 26) Sulfur and oxygen react to produce sulfur trioxide. In a particular experiment, 7.9 grams of SO3 are produced by the reaction of 5.0 grams of O2 with 6.0 grams of S. What is the % yield of SO3 in this experiment? A) 32 B) 63 C) 75 D) 95 E) 99 Answer: D