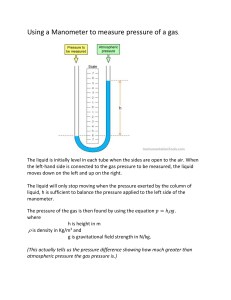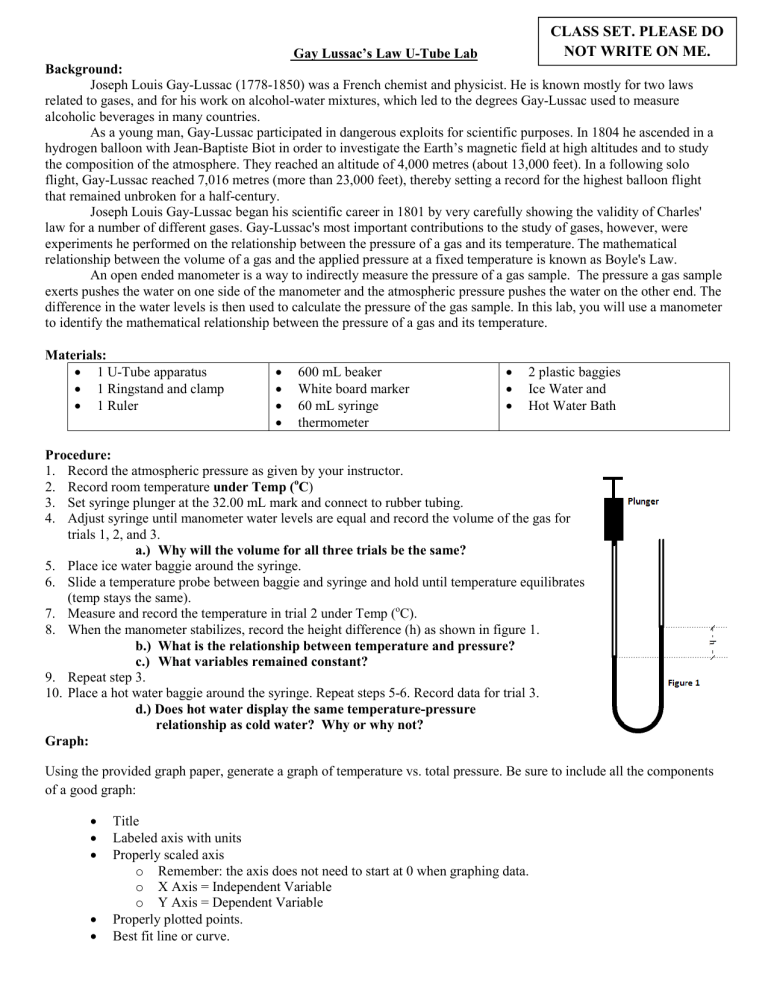
CLASS SET. PLEASE DO NOT WRITE ON ME. Gay Lussac’s Law U-Tube Lab Background: Joseph Louis Gay-Lussac (1778-1850) was a French chemist and physicist. He is known mostly for two laws related to gases, and for his work on alcohol-water mixtures, which led to the degrees Gay-Lussac used to measure alcoholic beverages in many countries. As a young man, Gay-Lussac participated in dangerous exploits for scientific purposes. In 1804 he ascended in a hydrogen balloon with Jean-Baptiste Biot in order to investigate the Earth’s magnetic field at high altitudes and to study the composition of the atmosphere. They reached an altitude of 4,000 metres (about 13,000 feet). In a following solo flight, Gay-Lussac reached 7,016 metres (more than 23,000 feet), thereby setting a record for the highest balloon flight that remained unbroken for a half-century. Joseph Louis Gay-Lussac began his scientific career in 1801 by very carefully showing the validity of Charles' law for a number of different gases. Gay-Lussac's most important contributions to the study of gases, however, were experiments he performed on the relationship between the pressure of a gas and its temperature. The mathematical relationship between the volume of a gas and the applied pressure at a fixed temperature is known as Boyle's Law. An open ended manometer is a way to indirectly measure the pressure of a gas sample. The pressure a gas sample exerts pushes the water on one side of the manometer and the atmospheric pressure pushes the water on the other end. The difference in the water levels is then used to calculate the pressure of the gas sample. In this lab, you will use a manometer to identify the mathematical relationship between the pressure of a gas and its temperature. Materials: • 1 U-Tube apparatus • 1 Ringstand and clamp • 1 Ruler • • • • 600 mL beaker White board marker 60 mL syringe thermometer • • • 2 plastic baggies Ice Water and Hot Water Bath Procedure: 1. Record the atmospheric pressure as given by your instructor. 2. Record room temperature under Temp (oC) 3. Set syringe plunger at the 32.00 mL mark and connect to rubber tubing. 4. Adjust syringe until manometer water levels are equal and record the volume of the gas for trials 1, 2, and 3. a.) Why will the volume for all three trials be the same? 5. Place ice water baggie around the syringe. 6. Slide a temperature probe between baggie and syringe and hold until temperature equilibrates (temp stays the same). 7. Measure and record the temperature in trial 2 under Temp (oC). 8. When the manometer stabilizes, record the height difference (h) as shown in figure 1. b.) What is the relationship between temperature and pressure? c.) What variables remained constant? 9. Repeat step 3. 10. Place a hot water baggie around the syringe. Repeat steps 5-6. Record data for trial 3. d.) Does hot water display the same temperature-pressure relationship as cold water? Why or why not? Graph: Using the provided graph paper, generate a graph of temperature vs. total pressure. Be sure to include all the components of a good graph: • • • • • Title Labeled axis with units Properly scaled axis o Remember: the axis does not need to start at 0 when graphing data. o X Axis = Independent Variable o Y Axis = Dependent Variable Properly plotted points. Best fit line or curve. Name: Gay Lussac’s Law U-Tube Lab Purpose: What is the relationship amongst the temperature and pressure of a gas? IV: DV: Procedural Questions: a.) Why will the volume for all three trials be the same? b.) What is the relationship between temperature and pressure? c.) What variables remained constant? d.) Does hot water display the same temperature-pressure relationship as cold water? Why or why not? Data: Atmospheric Pressure (mm Hg) _________________________ Trial Gas sample 1 Ice Water 2 Room Temp 3 Hot Water Temp. (o C) Gas Volume (mL) Height Difference (mm of H2O) 0.0 Calculations: • Record all answers in the space provided. Show your work below the table. 1. Calculate the temperature in Kelvin (K), using the following equation: K = oC + 273.2 2. Convert your Height Difference in mm H2O data into mm Hg for each of your temperature values: 1 mm Hg = 13.6 mm H2O 3. Total pressure (in mm Hg) = height difference (in mm Hg) + atmospheric pressure (mm Hg). Trial Gas sample 1 Ice Water 2 3 Room Temp Hot Water Temp (K) Height Difference: (mm Hg) Total pressure (mm Hg) Graph: Temperature (Kelvin) vs. Total Pressure Conclusion: • Summary o What is the relationship between the temperature of a gas and its pressure (direct or inverse proportionality)? • Purpose Analysis o What is the mathematical relationship between the temperature of a gas and its pressure? Give the equation. o Support your answer with data from the lab. • Error Analysis o For this lab, we assumed that the volume of the gas and amount of gas in the syringe remained constant. How would changing the volume of the gas or amount of the gas in the syringe impact your results? o What steps did we take to make sure this did not happen? • Application o Using Gay Lussac’s Law, explain why an aerosol can should be kept away from open flames.