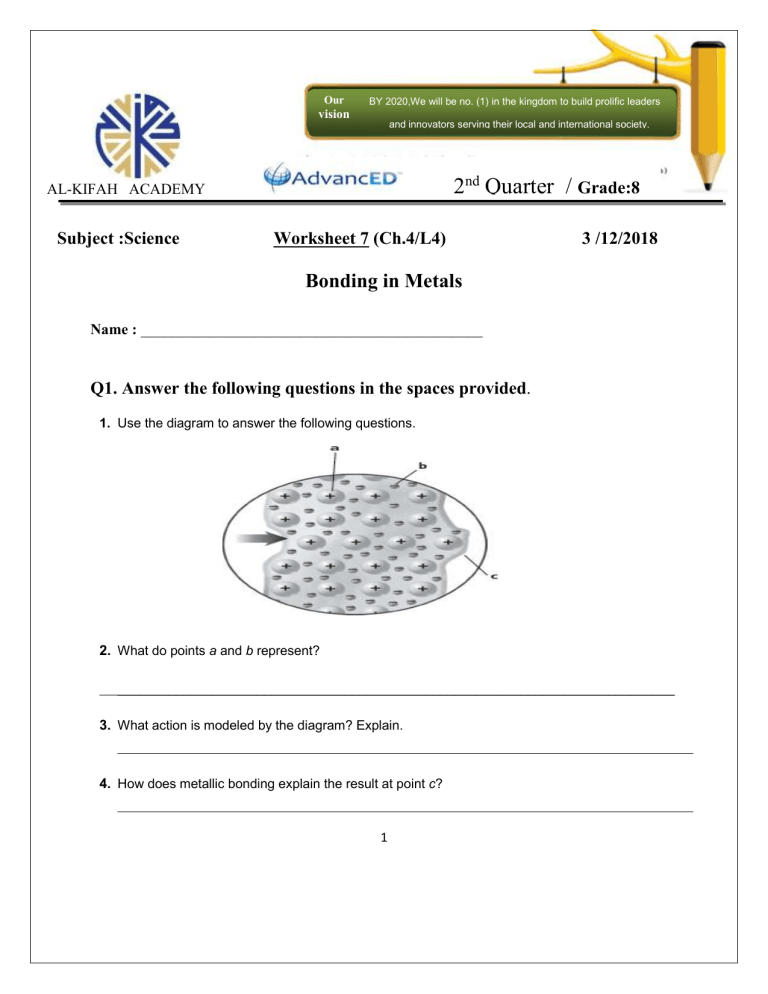
Our BY 2020,We will be no. (1) in the kingdom to build prolific leaders vision and innovators serving their local and international society. 2nd Quarter / Grade:8 AL-KIFAH ACADEMY 44 Subject :Science Worksheet 7 (Ch.4/L4) 3 /12/2018 Bonding in Metals Name : _____________________________________________ Q1. Answer the following questions in the spaces provided. 1. Use the diagram to answer the following questions. 2. What do points a and b represent? ____________________________________________________________________________ 3. What action is modeled by the diagram? Explain. 4. How does metallic bonding explain the result at point c? 1 5. write a definition for each of these terms. a. metallic bond : b. alloy : ______________________________________________________________________ Q2: Match each property of metal with its description by writing the letter of the correct description in the right column on the line beside the property in the left column. 1. luster a. easily beaten into complex shapes 2. ductility b. conducts electric current well 3. malleability c. shiny and reflective 4. thermal conductivity d. easily bent and pulled into thin strands 5. electrical conductivity e. conducts heat well Q3: Write the letter of the correct answer on the line at the left. 1. A B C D Why are alloys generally used to make everyday objects? Alloys are often stronger and less reactive than pure metals. Alloys have higher melting points than pure metals. Alloys are less expensive to produce than pure metals. Alloys have ionic bonds instead of metallic bonds. A B C D Metallic bonding is a type of covalent bond a type of ionic bond an attraction between positive and negative ions an attraction between positive ions and electrons A B C D Which of the following is NOT a property of metals? ductile good electrical conductor good thermal insulator malleable 2. 3. 2 4. A B C D At room temperature, most metals are liquid solid gas an alloy Q4 : Fill in the blank to complete each statement. 5. An attraction between a positive metal ion and surrounding electrons is a(n) _______________ bond. 6. Metals typically have melting points. 7. The metal fins that cool a motorcycle’s engine make use of the high________________ conductivity of metals. 8. Metals are often used to make wire because they are 9. Metals are used in electrical wires because they have high 10. Nonmetals are unlikely to form metallic bonds because their are strongly held. Science Teacher Mrs. Maryam Alabdulqader Academic Supervisor Mrs.Noha Ahmed Principal Mrs. Reem Al Mousa 3 . conductivity.

