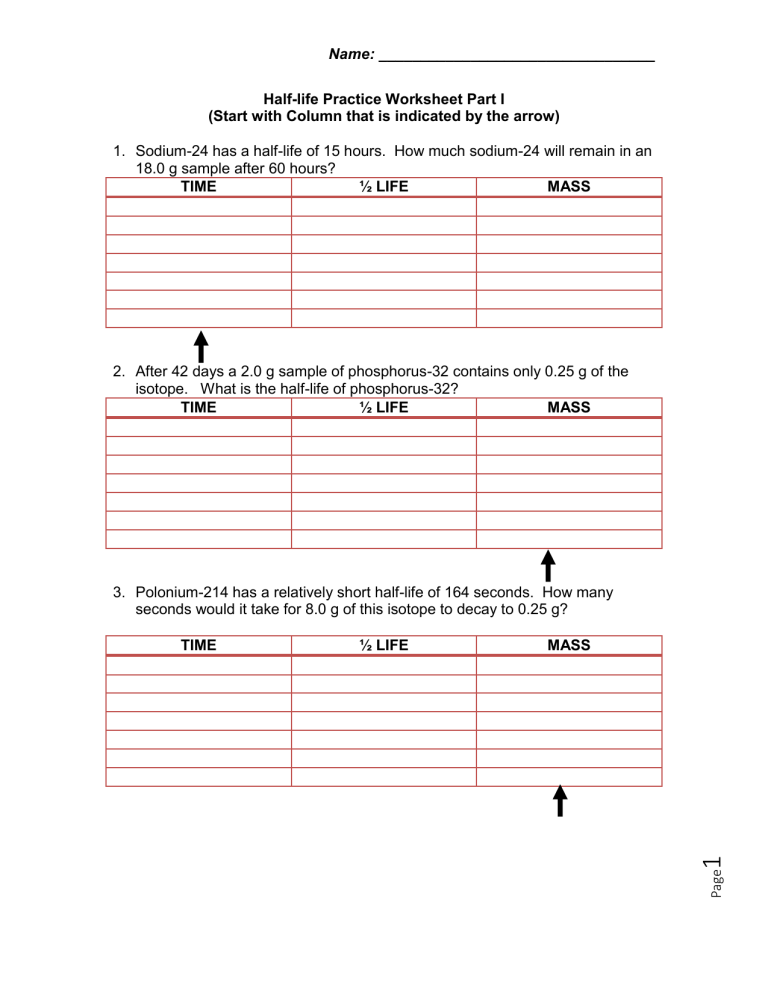
Name: _________________________________ Half-life Practice Worksheet Part I (Start with Column that is indicated by the arrow) 1. Sodium-24 has a half-life of 15 hours. How much sodium-24 will remain in an 18.0 g sample after 60 hours? TIME ½ LIFE MASS 2. After 42 days a 2.0 g sample of phosphorus-32 contains only 0.25 g of the isotope. What is the half-life of phosphorus-32? TIME ½ LIFE MASS 3. Polonium-214 has a relatively short half-life of 164 seconds. How many seconds would it take for 8.0 g of this isotope to decay to 0.25 g? MASS 1 ½ LIFE Page TIME Name: _________________________________ 4. How many days does it take for 16 g of palladium-103 to decay to 1.0 g? The half-life of palladium-103 is 17 days. TIME ½ LIFE MASS 5. In 5.49 seconds, 1.20 g of argon-35 decay to leave only 0.15 g. What is the half-life of argon-35? TIME ½ LIFE MASS Half life = decay time ÷ # of half lives 6. The half-life of Cs-137 is 30.2 years. If the initial mass of the sample is 1.00kg, how much will remain after 151 years? MASS 2 ½ LIFE Page TIME Name: _________________________________ 7. Carbon-14 has a half-life of 5730 years. Consider a sample of fossilized wood that when alive would have contained 24g of C-14. It now contains 1.5g. How old is the sample? TIME ½ LIFE MASS 8. A 64g sample of Germanium-66 is left undisturbed for 12.5 hours. At the end of that period, only 2.0g remain. What is the half-life of this material? TIME ½ LIFE MASS Page 3 Half life = decay time ÷ # of half lives Name: _________________________________ Solutions: Half-life Practice Worksheet Part I (Start with Column that is indicated by the arrow) 1. Sodium-24 has a half-life of 15 hours. How much sodium-24 will remain in an 18.0 g sample after 60 hours? TIME ½ LIFE MASS 0 0 18 15 1 9 30 2 4.5 45 3 2.25 60 4 1.13 2. After 42 days a 2.0 g sample of phosphorus-32 contains only 0.25 g of the isotope. What is the half-life of phosphorus-32? TIME ½ LIFE MASS 0 0 2 14 1 1 28 2 .5 42 3 .25 3. Polonium-214 has a relatively short half-life of 164 seconds. How many seconds would it take for 8.0 g of this isotope to decay to 0.25 g? MASS 8 4 2 1 .5 .25 4 ½ LIFE 0 1 2 3 4 5 Page TIME 0 164 328 492 656 820 Name: _________________________________ 4. How many days does it take for 16 g of palladium-103 to decay to 1.0 g? The half-life of palladium-103 is 17 days. ½ LIFE 0 1 2 3 4 TIME 0 17 34 68 68 MASS 16 8 4 2 1 5. In 5.49 seconds, 1.20 g of argon-35 decay to leave only 0.15 g. What is the half-life of argon-35? ½ LIFE TIME 0 1.83 3.66 5.49 0 1 2 3 MASS 1.2 .6 .3 .15 ½ LIFE 0 1 2 3 4 5 MASS 1.0 .5 .25 .125 .0625 .03125 Page TIME 0 30.2 60.4 90.6 120.8 151 5 6. The half-life of Cs-137 is 30.2 years. If the initial mass of the sample is 1.00kg, how much will remain after 151 years? Name: _________________________________ Half life = decay time ÷ # of half lives 7. Carbon-14 has a half-life of 5730 years. Consider a sample of fossilized wood that when alive would have contained 24g of C-14. It now contains 1.5g. How old is the sample? TIME 0 5730 11460 17190 22920 ½ LIFE 0 1 2 3 4 MASS 24 12 6 3 1.5 8. A 64g sample of Germanium-66 is left undisturbed for 12.5 hours. At the end of that period, only 2.0g remain. What is the half-life of this material? TIME 0 2.5 5.0 7.5 10.0 12.5 ½ LIFE 0 1 2 3 4 5 MASS 64 32 16 8 4 2 Page 6 Half life = decay time ÷ # of half lives




