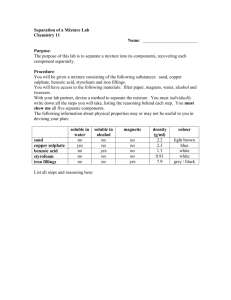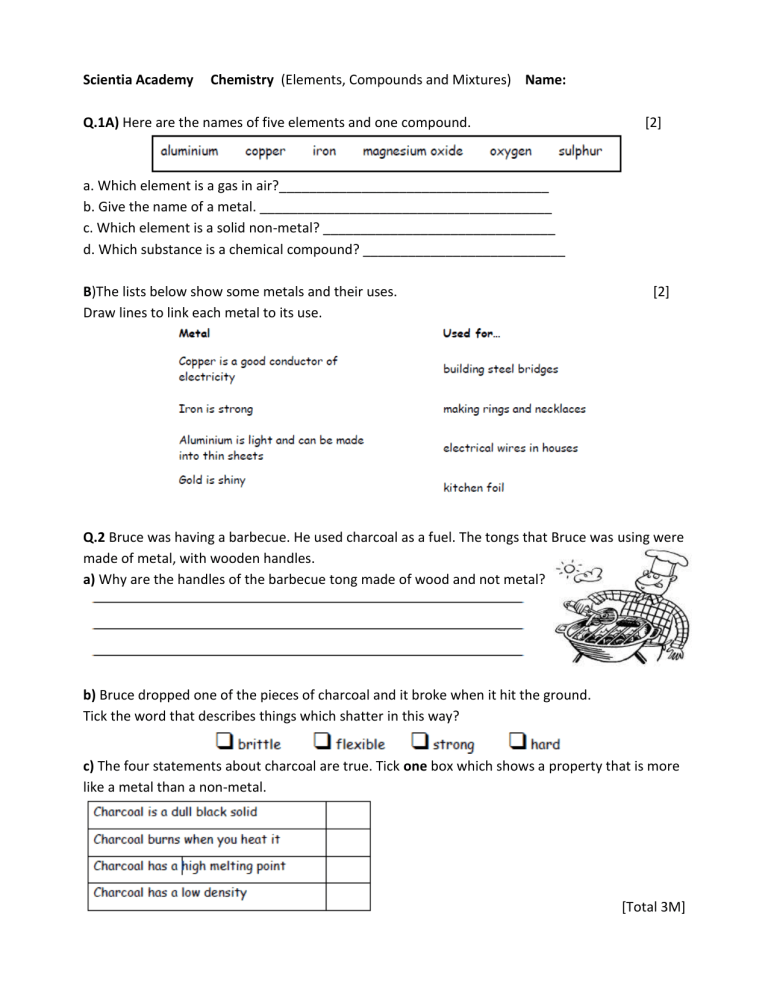
Scientia Academy Chemistry (Elements, Compounds and Mixtures) Name: Q.1A) Here are the names of five elements and one compound. [2] a. Which element is a gas in air?____________________________________ b. Give the name of a metal. _______________________________________ c. Which element is a solid non-metal? _______________________________ d. Which substance is a chemical compound? ___________________________ B)The lists below show some metals and their uses. Draw lines to link each metal to its use. [2] Q.2 Bruce was having a barbecue. He used charcoal as a fuel. The tongs that Bruce was using were made of metal, with wooden handles. a) Why are the handles of the barbecue tong made of wood and not metal? b) Bruce dropped one of the pieces of charcoal and it broke when it hit the ground. Tick the word that describes things which shatter in this way? c) The four statements about charcoal are true. Tick one box which shows a property that is more like a metal than a non-metal. [Total 3M] Q.3 Samantha opened a tin of white paint. The paint consisted of a liquid and particles of titanium dioxide that are insoluble in the liquid. The paint had separated into two layers, as shown below. liquid particles of insoluble titanium dioxide (i) What type of substance is the paint? Tick the correct box. a compound an element a mixture (ii) What type of substance is titanium dioxide? Tick the correct box. a compound an element a mixture (iii) Why did the particles of insoluble titanium dioxide sink to the bottom? [Total 3M] Q.4 Copper can be obtained from its ore, copper sulphide, in two stages. First stage heating the ore in air Copper sulphide reacts with oxygen from the air to form copper oxide and sulphur dioxide gas. Second stage heating the copper oxide with carbon Copper oxide reacts with carbon to form copper and carbon dioxide gas. (a) Give the names of three elements mentioned above. 1. ................................................................................................................. 2. ................................................................................................................. 3. ................................................................................................................. (b) Give the name of one compound mentioned above. (c) Give the name of the compound, mentioned above which causes ‘acid rain’. [Total 5M] Q.5 Chris collected some sea water near a beach. The sea water had salt dissolved in it. It had sand mixed in it. (a) Chris separated the sand from the salt water as shown below. (i) What is this method of separation called? Tick the correct box. (ii) What is substance A? (iii) What is the part labelled B? (b) Chris poured some of the salt water from the flask into a dish. He put the dish on a balance and left it in a warm room for a week. (ii) After one week there was a white solid but no liquid in the dish. What had happened to the water in the dish? (iii) What was the white solid left in the dish? [Total 5M] Q.6) A teacher mixed iron filings with sulphur on a metal tray. She heated the mixture in a fume cupboard. Sulphur is yellow. Iron filings are grey. The mixture glowed very brightly. The teacher turned off the bunsen burner. The glow spread through the mixture. When the mixture cooled, a black solid called iron sulphide was left. (a) From this information, give one way you can tell that a chemical reaction took place. (b) What type of substance is each of the chemicals involved in this reaction? Choose from: (c) Raj held a magnet near to each of the three chemicals. By each chemical in the table, write yes or no to show if the chemical was magnetic. [Total 6M] Q.7) Meera added blue copper sulphate crystals to some water in a beaker. The copper sulphate dissolved in the water. (i) Give one way Meera could see that the copper sulphate had dissolved in the water. ___________________________________________________________________ (ii) Give one way that she could get the copper sulphate to dissolve more quickly. ___________________________________________________________________ (d) Meera poured some of the copper sulphate solution into a dish. She left it in a warm room for a week. A week later there was a blue solid but no liquid in the dish. (i) What happened to the water in the copper sulphate solution? ___________________________________________________________________ (ii) What was the blue solid left in the dish? ______________________________ Q.8) Rema used the apparatus below to distil 100 cm3 of water-soluble ink. (a) Which processes occur during distillation? Tick the correct box. (b) Give the name of the colourless liquid that collects in the test-tube. [Total 4M] (c) What would the temperature reading be on the thermometer when the ink has been boiling for two minutes? _______°C (d) (i) Water at 15°C enters the condenser at X. Predict the temperature of the water when it leaves the condenser at Y. _______°C Explain this change of temperature. (ii) Give two ways in which the water vapour changes as it passes down the glass tube in the condenser. 1. ___________________________________________________ 2. ___________________________________________________ (e) Peter used the apparatus below to distil 100 cm3 of water-soluble ink. Why is the condenser in apparatus A better than the glass tube and beaker of water in apparatus B? [Total 6M] Q.9) (a) Reshma had a mixture of iron filings and sand. What could she use to separate the iron filings from the mixture? (b) Reshma put 10 cm3 of water and 2 g of a different solid into each of four test-tubes. She shook each test-tube. The drawings show the test-tubes after 10 minutes. Why can the salt and sugar no longer be seen in test-tubes A and C? [Total 2M] Q.10) A meteorite landed on Earth. It contained a new element. Scientists called the element jovium. (a) The list below shows some properties of jovium. Which two properties suggest that jovium could be a metal? Tick two boxes. (b) A scientist put a piece of the meteorite in water and stirred it. This produced a blue solution with tiny, solid, black particles in it. He separated the black particles from the blue solution using the apparatus below. (i) Give the name of this method of separation. (ii) The diagram below shows the results. What do the labels A and B show? Write your answers on the lines. (c) The scientist poured the contents of the flask into a dish. Two days later there were blue crystals in the dish, but no liquid. What happened to the liquid in the dish? [Total 4M]