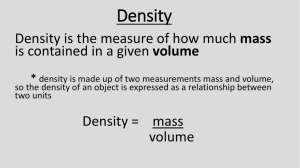DENSITY BLOCKS – Lab Activity Name ____________________________ Table # ______ 1. Describe the appearance of each block 2. Predict if each will sink of float, then test if each sinks of floats 3. For those that sink find the displacement (final volume – initial volume) Density Description Block (color, what it looks like) A B C D E F G H Float prediction (yes or no) Does it float (yes or no) Initial water volume (mL) Final water volume (mL) Displacement (mL) 1. Were your predictions about floating accurate? 2. If the cubes don’t sink, what is another way we could calculate the volume? Activity 2 1. Find the mass of each block using a triple beam balance 2. Find the mass of each block using an electric balance 3. Find the volume for each block using the formula L x W x H in cm! 4. Find the density of each block using the formula D= M/V Density Description (color, Block what it looks like) A B C D E F G H Mass (g) – triple beam balance Mass (g) – electric balance (use this to determine final density) Volume (cm3) Density (g/cm3) 1. Compare the volume of each block. What pattern did you notice? _____________________________________________________________________________________ 2. Is this same pattern evident for the density you calculated? _____________________________________________________________________________________ 6. Refer to the Density Chart (below). Do the densities you calculated for each substance match any of the numbers listed in the chart? Which materials can you identify? List the blocks in order of density from least dense to most dense. least dense substance description, density most dense substance description, density Density Chart Substance Acrylic Aluminum Brass Copper Oak Pine Density (g/cm3) 1.1-1.2 2.7 8.5 8.9 0.6-0.9 0.45 Polypropylene PVC Steel Water 0.9-0.92 1.4 7.9 1.0 ACTIVITY 3 1. Obtain three blocks of the same substance (with the same letter on them).Record the name of the substance (from above) in the appropriate space in Data Table 3. 2. Measure the mass of one block to the nearest tenth of a gram using an electronic scale or triple beam balance. Record the mass in the appropriate space in table 3. 3. Calculate the volume of the block by multiplying the length (cm) by the width (cm) by the height (cm). Record this volume in the appropriate space in Data Table 3. 4. Measure the mass of two blocks to the nearest tenth of a gram. Record this combined mass in the appropriate space in Data Table 3. 5. Place two blocks next to each other. Calculate the volume of this new combined block by measuring and multiplying the length by the width by the height. Record this combined volume in the appropriate space in Data Table 3. 6. Repeat steps 5 and 6 using three blocks. 7. Repeat steps 5 and 6 using 3 blocks. (Note: You may place 3 blocks four in a row, or two on top of each other. Either way the volume is the same.) Data Table 3 Description of Substance: _______________________________ Letter of block:____________ Mass (g) One Cube Two Cubes Three Cubes Volume (cm3) Density (g/cm3) 8. Make a line graph! Plot your data for the cubes from Activity 3 on the graph below. Be sure to mark the numerical units for your grid lines on each axis. Mass (g) Volume (cm3) 9. Using information from your graph, determine the correct answer to each of the following questions: A. As volume increases, mass (increases, decreases, remains the same). B. As volume decreases, mass (increases, decreases, remains the same). C. What is the formula for calculating density? D. Compare the slope of your line to the density of the substance you measured. Are these two numbers similar or not? Why or why not? E. As mass and volume increase, density (increases, decreases, remains the same). F. As mass and volume decrease, density (increases, decreases, remains the same).