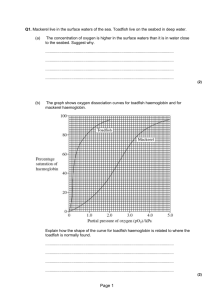
Explain oxygen dissociation for adult and foetal haemoglobin and myoglobin Know the structure of haemoglobin amd myoglobin Know what is dissociation curve Investigate dissociation curves for haemoglobin and myoglobin Describe the structure of haemoglobin and myoglobin Write the differences between two of them Haemoblobin Structure Description Myoglobin The oxygen dissociation curve is a graph that plots the proportion of haemoglobin in its oxygen-laden saturated form on the vertical axis against the partial pressure of oxygen on the horizontal axis. The curve is a valuable aid in understanding how the blood carries and releases oxygen and it is a common theme that is tested on in many medical examinations. At high partial pressures of oxygen, haemoglobin binds to oxygen to form oxyhaemoglobin. All of the red blood cells are in the form of oxyhaemoglobin when the blood is fully saturated with oxygen. Each gram of haemoglobin can combine with 1.34 mL of oxygen. At low partial pressures of oxygen (e.g. within tissues that are deprived of oxygen), oxyhaemoglobin releases the oxygen to form haemoglobin. The oxygen dissociation curve can be shifted right or left by a variety of factors. A right shift indicates decreased oxygen affinity of haemoglobin allowing more oxygen to be available to the tissues. A left shift indicates increased oxygen affinity of haemoglobin allowing less oxygen to be available to the tissues. A decrease in the pH shifts the curve to the right, while an increase in pH shifts the curve to the left. This occurs because a higher hydrogen ion concentration causes an alteration in amino acid residues that stabilises deoxyhaemoglobin in a state (the T state) that has a lower affinity for oxygen. This rightwards shift is referred to as the Bohr effect. A decrease in CO2 shifts the curve to the left, while an increase in CO2 shifts the curve to the right. CO2 affects the curve in two ways. Firstly, accumulation of CO2 causes carbamino compounds to be generated, which bind to oxygen and form carbaminohaemoglobin. Carbaminohaemoglobin stabilizes deoxyhaemoglobin in the T state. Secondly, accumulation of CO2 causes an increase in H+ ion concentrations and a decrease in the pH, which will shift the curve to the right as explained above. An increase in temperature shifts the curve to the right, whilst a decrease in temperature shifts the curve to the left. Increasing the temperature denatures the bond between oxygen and haemoglobin, which increases the amount of oxygen and haemoglobin and decreases the concentration of oxyhaemoglobin. Temperature does not have a dramatic effect but the effects are noticeable in cases of hypothermia and hyperthermia. Factor Decrease Increase pH CO2 Temperature 2,3-DPG Right shift Left shift Left shift Left shift Left shift Right shift Right shift Right shift Fetal haemoglobin (HbF) is the main oxygen transport protein in the human fetus during the last 7 months of development. It persists in the newborn until roughly 6 months of age. HbF has different globin chains to adult haemoglobin (Hb). Whereas adult haemoglobin is composed of two alpha and two beta subunits, fetal haemoglobin is composed of two alpha and two gamma subunits. This change in the globin chain results in a greater affinity for oxygen and allows the fetus to extract blood from the maternal circulation. This increased affinity for oxygen means that the oxygen dissociation curve for fetal haemoglobin is shifted to the left of that of adult haemoglobin. The curve for myoglobin lies even further to the left than that of fetal haemoglobin and has a hyperbolic, not sigmoidal, shape. Myoglobin has a very high affinity for oxygen and acts as an oxygen storage molecule. It only releases oxygen when the partial pressure of oxygen has fallen considerably. The function of myoglobin is to provide additional oxygen to muscles during periods of anaerobic respiration.